Afebrile malaria patient with multisystem involvement and Hepatitis B infection: A case report
Rabindra Ghimire, Kaushal Raj Pandey, Prabhat Adhikari, Ashna Pokhrel, Maximo Mora and Mirela Sam
Cite this article as: BJMP 2012;5(2):a513
|
|
Abstract Malaria is caused by obligate intraerythrocytic protozoa of the genus Plasmodium. Humans can be infected with one (or more) of the following five species: P. falciparum, P. vivax, P. ovale, and P. malariae and P. knowlesi. Malaria typically produces a string of recurrent attacks, or paroxysms, each of which has three stages; chills, followed by fever, and then sweating. Although malaria without fever is rare,we present a complicated case of P. ovale malaria without fever associated with Hepatitis B virus infection, pre-excitation pattern ECG and secondary adrenal insufficiency in a young African American adult male who had travelled to Africa 9 months prior to clinical presentation. Our patient did not have any features to characterize severe malaria and the parasitemia was <5%. Keywords: Malaria, Plasmodium ovale, Preexcitation, Hepatitis B |
Malaria is caused by obligate intra-erythrocytic protozoa of the genus Plasmodium. Humans can be infected with one (or more) of the following five species: P. falciparum, P. vivax, P. ovale, and P. malariae and P. knowlesi. Plasmodia are transmitted by the bite of an infected female Anopheles mosquito and these patients commonly present with fever, headache, fatigue and musculoskeletal symptoms.
Diagnosis is made by demonstration of the parasite in peripheral blood smear. The thick and thin smears are prepared for identification of malarial parasite and genotype respectively. Rapid diagnosis of malaria can be done by fluorescence microscopy with light microscope and interference filter or by polymerase chain reaction.
We report a complicated case of P. ovale malaria without fever associated with Hepatitis B virus infection, pre-excitation (WPW pattern), and secondary adrenal insufficiency.
Case Report:
A 23 year old African American man presented to the emergency department with headache and dizziness for one week. He had 8/10 throbbing headaches associated with dizziness, nausea and ringing sensation in the ears and also complained of sweating but denied any fever. He had loose, watery bowel movements 3 times a day for a few days and had vomited once 5 days ago. He denied any past medical history or family history. He was a chronic smoker and smoked 1PPD for 8 years and denied alcohol or drug use. He had travelled to Africa 9 months before presentation and had stayed in Senegal for 1 month though he did not have any illnesses during or after returning from Africa.
On examination: T: 97.6, HR: 115/min, BP: 105/50, no orthostasis, SPO2: 100% in room air and RR: 18/min. Head, neck and throat examinations were normal and respiratory and cardiovascular system examinations were unremarkable except for tachycardia. Abdominal examination revealed no organomegaly and his CNS examination was unremarkable.
Laboratory examination revealed: WBC: 6.4, Hb: 14.4 and Hct: 41.3, Platelets: 43, N: 83.2, L: 7.4, M: 9.3, B: 0.1. His serum chemistry was normal except for a creatinine of 1.3 (BUN 14) and albumin of 2.6 (total protein 5.7). A pre-excitation (WPW Pattern) was seen on ECG and head CT and Chest X-ray were normal.
He was admitted to the telemetry unit to monitor for arrhythmia. Peripheral blood smear (PBS) was sent because of thrombocytopenia and mild renal failure and revealed malarial parasites later identified as P. ovale (Pic. 1 and 2).
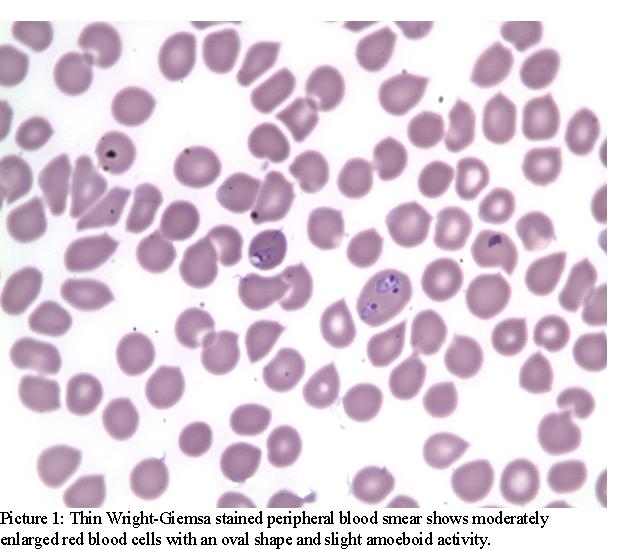
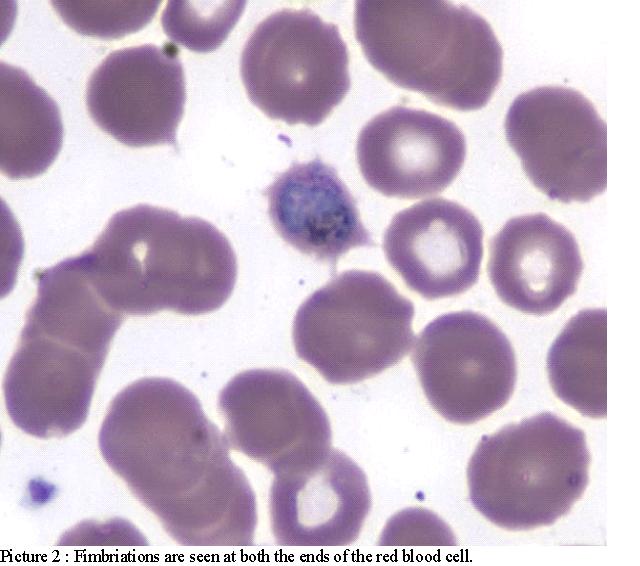
He was treated with Malarone; yet after 2 days of treatment, he was still complaining of headache, nausea and dizziness. There were no meningeal signs. His blood pressure readings were low (95/53) and he was orthostatic. His ECG showed sinus tachycardia and did not reveal any arrhythmias or QTc prolongation. His morning serum cortisol was 6.20 and subsequent cosyntropin stimulation test revealed a serum cortisol of 13.40 at one hour after injection. His Baseline ACTH was<1.1 suggesting a secondary adrenal insufficiency. His IGF-1, TSH, FT4, FSH, LH were all within normal limits. His bleeding and coagulation parameters were normal, CD4 was 634(CD4/CD8: 1.46) and rapid oral test for HIV was negative. His Hepatitis B profile was as follows: HBsAg: positive, HBV Core IgM: negative, HBV core IgG: positive, HBeAg: negative, HBeAb: positive, HBV DNA: 1000 copies/ml, Log10 HBV DNA: 3000 copies/ml.
His Blood cultures were negative, his G6PD levels and hemoglobin electrophoresis were normal, haptoglobin was<15 and LDH was 326. MRI of the brain was unremarkable. The abdominal sonogram revealed a normal echo pattern of the liver and spleen and spleen size was 12 cm. The secondary adrenal insufficiency was treated with dexamethasone resulting in gradual improvement of his nausea, vomiting and headache. Furthermore the platelet count improved to 309. Primaquine was prescribed to complete the course of malaria treatment and he was discharged home following 8 days of hospitalization. Unfortunately he did not return for follow up.
Discussion:
Malaria continues to be a major health problem worldwide. In 2007 the CDC received reports of 1,505 cases of malaria among person in the United States. 326 cases were reported from New York with all but one of these cases being acquired outside of the United States1.
While Plasmodia are primarily transmitted through the bite of an infected female Anopheles mosquito, infections can also occur through exposure to infected blood products (transfusion malaria) or by congenital transmission. In industrialized countries most cases of malaria occur among travellers, immigrants, or military personnel returning from areas endemic for malaria (imported malaria). Exceptionally, local transmission through mosquitoes occurs (indigenous malaria). For non-falciparum malaria the incubation period is usually longer (median 15–16 days) and both P. Vivax and P. Ovale malaria may relapse months or years after exposure due to the presence of hypnozoites in the liver of which the longest reported incubation period for P. vivax being 30 years2.
Malaria without fever has been reported in cases of Plasmodium falciparum malaria in non- immune people3. Hepatitis B infection associated with asymptomatic malaria has been reported in the Brazilian Amazon4. This study was done in P. falciparum and P. vivax infected person with HBV co-infection though not in the P. ovale group. HBV infection leads to increased IFN-gamma levels5,6 which are important for plasmodium clearance in the liver7, in addition to its early importance for malarial clinical immunity8. High levels of IFN gamma, IL6 and TNF alpha are detectable in the blood of malaria patients and in the spleen and liver in the rodents’ model of malaria9,10. These inflammatory cytokines are known to suppress HBV replication in HBV transgenic mice9. This might explain the low levels of HBV viremia in our patient although human studies are required to confirm this finding.
The hypothalamic-pituitary- adrenocortical axis suppression and primary and secondary adrenal insufficiency has been reported in severe falciparum malaria10. In our case, the patient did not have any features to characterize severe malaria, and parasitaemia was <5%. Further, the MRI did not reveal any secondary cause for adrenal insufficiency. This might indicate that patients with malaria are more prone for hypothalamo-pituitary adrenocortical axis dysregulation yet further studies are required to prove this phenomenon in patients without severe malaria.
Cardiac complications after malaria have rarely been reported. In our patient pre-excitation on ECG disappeared after starting antimalarial treatment. Whether WPW pattern and its subsequent disappearance was incidental or caused by malarial infection that improved with treatment could not be determined. Lengthening of the QTc and severe cardiac arrhythmia has been observed, particularly after treatment with halofantrine for chloroquine resistant Plasmodium falciparum malaria11. Post-infectious myocarditis can be associated with cardiac events especially in combination with viral infections12. A case of likely acute coronary syndrome and possible myocarditis was reported after experimental human malaria infection13. To date, except for cardiac arrhythmias that developed after treatment with halofantrine and quinolines, no other arrhythmias has been reported in patients with malaria before treatment.
Transient thrombocytopenia is very common in uncomplicated malaria in semi -immune adults14. A person with a platelet count <150 × 109/l is 4 times more likely to have asymptomatic malarial infection than one with a count ≥150 × 109/l15. In an observational study among 131 patients, patients with involvement of more than one organ system was found to have a lower mean platelet count compared to those with single organ involvement16.
Conclusions:
Our case highlights the need for further studies to understand the multi-organ involvement in patients without severe malaria as well as early recognition of potential complications to prevent mortality and morbidity in this subgroup of patients.
|
Acknowledgements We are thankful to our pathologist Maximo Mora, MD for providing the picture of the malarial parasites from our patient. Competing Interests None declared Author Details Rabindra Ghimire, MD: Resident, Internal Medicine PGY3, Interfaith Medical Center, Brooklyn, NY. Kaushal Raj Pandey, MD:Resident, Internal Medicine PGY3, Interfaith Medical Center, Brooklyn, NY. Prabhat Adhikari, MD: Resident, Internal Medicine PGY3, Interfaith Medical Center, Brooklyn, NY. Ashna Pokhrel, MBBS: Resident, Internal Medicine, Interfaith Medical Center, Brooklyn, NY. Maximo Mora, MD: Pathologist,Interfaith Medical Center, Brooklyn, NY. Mirela Sam, MD: Chief- Infectious Disease division, Interfaith Medical Center, Brooklyn, NY. CORRESPONDENCE: Rabindra Ghimire, MD Department of Medicine, 1545 Atlantic Avenue, Brooklyn, NY-11213 Email: drrabindraghimire@gmail.com |
References

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




