Analgesia in day-case ENT surgery: The efficacy of lornoxicam
Mohamed Daabiss, Medhat Al-Sherbiny, Rashed Al-Otaibi and Rima Al-Nimar
Cite this article as: BJMP 2009: 2(3) 46-50
|
|
Abstract Objectives: As pain management is important to facilitate early mobilization after surgery, which in turn results in a shorter hospital stay since early discharge and patient satisfaction are important goals in day-case surgery. The aim of this study was to demonstrate the perioperative analgesic efficacy of lornoxicam in minor to moderate day-case ENT surgical procedures. |
Introduction
Day-case surgery is of great value to patients and the health service. It has rapidly expanded as a cost-effective and resource-conserving surgical intervention. However, the ability to deliver a safe and cost-effective general anesthetic with minimal side effects and rapid recovery is demanded in a day-case surgery unit. Pain and emesis are the two major complaints after day case surgery. Opioids are the agents of choice for severe pain. However, this class of analgesics is associated with dose-dependent adverse effects such as PONV, sedation, respiratory depression, resulting in delayed discharge or prolonged hospital stay. Non-opioid analgesics, e.g. acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), are often used alone or as adjuncts to opioids because of fewer adverse effects compared to opioids alone. However, NSAIDs also have side effects [1]. Lornoxicam is a new NSAID that belongs chemically to oxicams, a chemical class including piroxicam and tenoxicam. Lornoxicam is a potent inhibitor of cyclo-oxygenase and the only oxicam with a 15 times shorter half-life than piroxicam and tenoxicam [2]. In addition, lornoxicam can be given by I.V. route. Lornoxicam has a better safety profile than diclofinac and naproxen with regards to renal and hepatic function tests. In addition to better GIT tolerability compared to selective COX2 inhibitors; it is completely metabolized to inactive metabolites [2,3]. Lornoxicam has been successfully used in prevention and treatment of postoperative pain. However, evaluation of the perioperative analgesic efficacy of lornoxicam in day-case surgery has not yet been studied. This randomized, double blinded study was designed to compare the quality of perioperative analgesia as well as side effects of IV lornoxicam versus fentanyl in patients scheduled for minor to moderate day-case ENT surgical procedures. Materials and Methods: Male or female patients (aged 18-60 yr) were eligible for inclusion in the study. After obtaining the approval of the Hospital Research & Ethical Committee and patient’s informed consent, patients were randomized into three groups of ASA class I and II, scheduled to undergo minor to moderate day-case ENT surgical procedures e.g. tonsillectomy, excision of ENT lesion (e.g. vocal cord nodules and cysts), polypectomy and endoscopic sinus operations were enrolled in this randomized, double blinded study between May and December 2008. Exclusion criteria were patients with body mass index (BMI) > 30%, drug or alcohol abuse, and known allergy to NSAIDs, paracetamol or any contraindications for opioid use. The protocol was similar for all patients. Prior to surgery, patients were educated in the use of the 10 – point visual analog scale (VAS) for pain assessment (0 = no pain to 10 = maximum pain). No premedication was given. In the holding area, an IV cannula was inserted and an IV infusion of Lactated Ringer’s was started. HR, MAP and SpO2 were recorded before induction (baseline value). Since fentanyl is a clear fluid while lornoxicam is yellow, the pharmacist prepared, covered and coded the medications in two coded envelopes for each patient. One envelope containing lornoxicam 8mg (L8), 16 mg (L16) or placebo to be given half an hour before induction of anesthesia and another envelope with fentanyl 100 µg (F) or placebo to be given with induction i.e. each patient received either IV (F), (L8) or (L16).The medications were administered by a different anesthetist, who was not involved in the study. Anesthesia was induced with propofol 2 mg/kg IV followed by cisatracurium 0.15 mg/kg IV to facilitate orotracheal intubation. After tracheal intubation, the patients were ventilated to normocapnia with sevoflurane (2-3% end tidal) in 50% oxygen in air. All patients received IV 1 gm of paracetamol after induction and were monitored with ECG, MAP, SpO2 and EtCO2. Supplementary fentanyl 0.5 µg/kg was given IV as required in all groups (if > 20% increases in MAP or HR than preinduction values in presence of adequate muscle relaxation). At the end of surgery, muscle relaxation was reversed and extubated. In the post-anaesthesia care unit (PACU), the time from extubation to spontaneous eye opening was compared between the groups. The patients were monitored with ECG, SpO2, MAP, respiratory rate (RR), VAS and sedation score (0 =awake, 1=mild sedation, 2=sleepy but arousable, and 3 = very sleepy) at 0.5,1, 2, 3 and 4 hours by an anaesthetist, who was not aware of the study drug used. Intramuscular (IM) injection of meperidine 1 mg/kg was administered as a rescue analgesic at VAS > 4. The total amount of meperidine required during first 4 hrs postoperative was recorded. The time of the study drugs injection was recorded after decoding their codes. The first need for rescue analgesic was recorded as the time from the administration of the study-drug and the administration of meperidine. The incidence of PONV or any adverse event was recorded. The PACU staff was not aware of the study drug given. The results were analyzed using SPSS version 16. Sample size was 35 patients for each group in order to detect a 20% change in HR and MAP. The α-error was assumed to be 0.05 and the type II error was set at 0.20. Numerical data were expressed as mean ± SD. The groups were compared with analysis of variances (ANOVA). The VAS pain scores were analyzed by Mann-Whitney U test. Categorical data were compared using the Chi square test. P value of 0.05 was used as the level of significance. Results 105 patients aged between 18 and 52 yr were enrolled in the study. There were no significant demographic differences between groups (Table 1). HR and MAP were significantly higher at 10 and 20 minutes after induction of anaesthesia in group L8 compared to groups F and L16 (P < 0.05) (Fig. 1,2). The number of patients with inadequate intra-operative analgesia was significantly higher in group L8 compared to groups F and L16 (Fig 3). In PACU, 40 minutes postoperatively, HR, MAP and VAS were significantly higher in groups F and L8 (Fig 4,5,6). The first analgesic requirement time was significantly longer in group L16 compared to groups F and L8 (Table 2). The mean sedation scores in PACU were insignificantly higher in groups F and L8 compared to group L16 (Table1). While the incidence of PONV was significantly higher in groups F and L8 (p<0.05) (Table 1). Table 1: Demographic characteristics, eye opening time, incidence of postoperative nausea and postoperative sedation score:
| F | L8 | L16 | P | |
| Age (year) - mean (range) | 31 (18-52) | 32 (18-51) | 31 (20-49) | 0.129 |
| Sex F/M | Oct-25 | Oct-25 | Sep-26 | 0.695 |
| Weight (Kg) | 72.7±11.7 | 74.1±11.3 | 75.3±9.9 | 0.402 |
| Height (cm) | 166.2±14.7 | 169.4±11.9 | 161±19.5 | 0.482 |
| ASA physical status I/II | 23-Dec | 22/13 | 25-Oct | 0.312 |
| Duration of surgery (min) | 58.8±21.8 | 59.6±21.4 | 56.9±23.3 | 0.675 |
| Time to eye opening (min) | 7.2±3.1 | 6.4±1.2 | 3.7±1.6* | 0.019* |
| Postoperative nausea | 9/35 | 7/35 | 3/35* | 0.002* |
| Postoperative sedation score (0 – 3) | 1.7±0.6 | 1.9±1.1 | 1.4±0.6 | 0.357 |
Data are expressed as mean ± SD or number of patients* Significant difference (P < 0.05). NS: Non significant.- Time to eye opening is the time from extubation to spontaneous eye opening. Table 2: Perioperative analgesic requirements and time to first postoperative analgesic requirement (mean ± SD)
| F | L8 | L16 | P | |
| Intra-operative fentanyl supplementation (µg) | 45.5 ± 13.2 | 67.8 ± 16.4* | 43.1 ± 10.2 | 0.012* |
| Time of 1st postoperative rescue analgesic (min) | 94.3 ± 33.4 | 101.6 ± 51.5 | 223.9 ± 62.3* | 0.0002* |
| Postoperative meperidine rescue (mg) | 76.3 ± 12.5 | 80.5 ± 11.7 | 39.9 ± 7.6* | 0.001* |
-Data are expressed as mean ± SD.* Significant difference (P < 0.05). NS: Non significant.-Time of 1st postoperative rescue analgesic is the time elapsed between the administration of the study drug and the administration of an analgesic postoperatively. 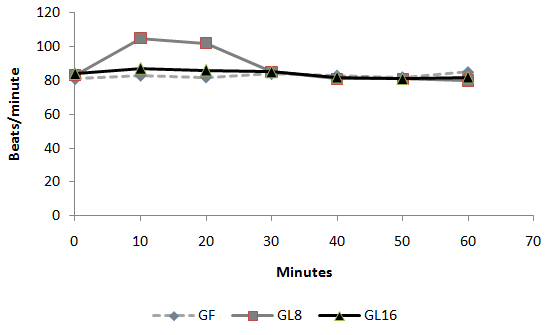 Fig 1: Intra-operative changes in heart rate in groups
Fig 1: Intra-operative changes in heart rate in groups 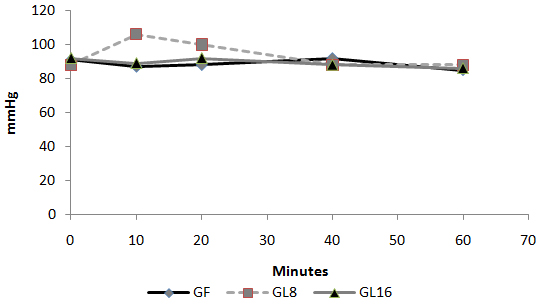 Fig 2: Intra-operative changes in MAP in groups
Fig 2: Intra-operative changes in MAP in groups 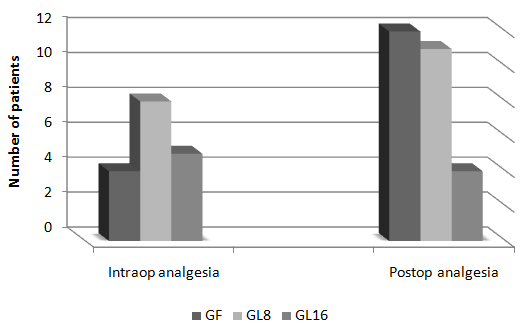 Fig 3: Number of patients requested perioperative analgesic supplementation
Fig 3: Number of patients requested perioperative analgesic supplementation 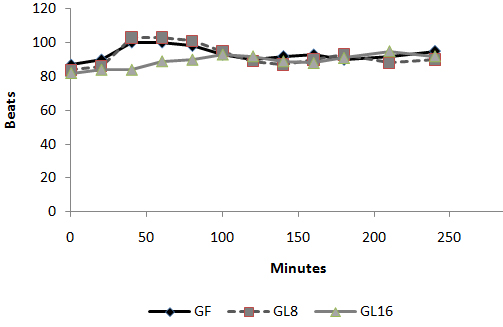 Fig 4: Changes in heart rate in PACU
Fig 4: Changes in heart rate in PACU 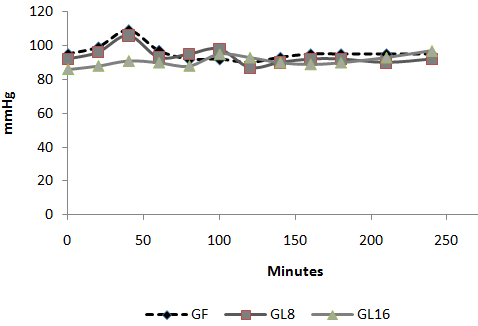 Fig 5: Changes in MAP in PACU:
Fig 5: Changes in MAP in PACU: 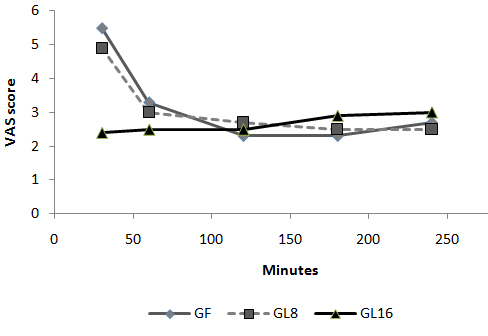 Fig 6: Changes in VAS in PACU Discussion: The use of an opioid, even a short acting one can be associated with adverse effects, which may not be acceptable for patients scheduled for day case surgery. For this reason, it was suggested to substitute an opioid with a non-opioid analgesic for postoperative pain control. The use of a NSAID is associated with adverse effects [1]. Lornoxicam has been successfully used in the prevention and treatment of postoperative pain. It has been shown to be as effective as morphine [4], meperidine [5] and tramadol [6]. To the best of our knowledge, this is the first study to compare the perioperative analgesic efficacy of lornoxicam to fentanyl in patients undergoing day case ENT surgery. We gave Lornoxicam half an hour before induction of anesthesia as the time taken to reach peak plasma concentration (Tmax) was determined to be 0.5 h [7]. During the operative procedure, HR and MAP were significantly higher in group L8 compared to group F and L16. While in PACU, patients in groups F and L8 had higher HR, MAP and VAS score in the early postoperative period compared to patients in group L16. This may be due to inadequate analgesic effect of L8 and the shorter plasma half life of fentanyl compared to L16. The analgesic efficacy of L16 might be attributable to inhibition of cyclo-oxygenase (COX1) and (COX2) activity [2], release of endogenous dynorphin and β-endorphin [5], decrease in peripheral and central prostaglandin production [8] as well as exertion of some of its analgesic activity via the central nervous system [9]. Lornoxicam has a more potent anti-inflammatory and analgesic effect than other oxicams as well as a shorter half life, which decreases the incidence of side effects of drugs with long plasma half life [10]. Arslan and colleagues reported decreased opioid need, PONV and postoperative pain scores when 16 mg of lornoxicam was administered after thyroidectomy [11]. While Xuerong and colleaguessuggested that the increase of postoperative morphine requirements induced by intra-operative administration of fentanyl could be prevented by ketamine or lornoxicam [12]. Rawal reported that NSAIDs are effective as the sole analgesic in a high proportion of cases of mild to moderate pain and it is more convenient to give these drugs by the IV route rather than by IM or rectal administration [13]. The analysis of pain intensity differences was complicated by the fact that many patients postoperatively were asleep at the time their pain assessments were due which may be attributed to effect of opioid and anesthetic medications used. To minimize any missing data we used time to the first dose rescue analgesia (based on changes in hemodynamic data) to evaluate pain intensity differences from baseline. L16 was well tolerated in this study, and was associated with a significantly lower incidence of adverse events than F and L8 which could be due to the opioid side effects in both groups. Norholt and colleaguessupported our results as they reported that, in terms of common acute adverse events, lornoxicam appeared to possess a higher benefit/risk ratio compared with morphine [4]. Zuurmond et al reported that, there is good evidence that avoidance of opioid virtually abolishes the PONV that preclude oral intake of fluids after surgery [14]. In our study, nausea developed in 25.7% of patients in group F, 20% in group L8 but only 8.6% in group L16 who received the least rescue opioid analgesia. Regarding bleeding abnormalities, Hodsman et al reported extensive bleeding required reoperation on two diclofenac group patients submitted to abdominoperitoneal resection of the rectum [15]. In our study no abnormal bleeding was reported by ENT surgeons in any of the study patients. In agreement with our results, Ilias et al[16], Trampitsch et al[17] and Karaman et al [18] used lornoxicam and they did not detect problems with surgical bleeding, bleeding time, blood transfusion requirement or postoperative bleeding. Stroissnig et al reported that overall, in healthy adult volunteers, oral doses of lornoxicam up to 70 mg have been well tolerated, and there have been no effects on vital signs, urine analysis parameters or clinical serum biochemistry [19]. In our study, none of the patients receiving study drugs experienced severe gastric discomfort, needed rescue antiemetic medication or required admission because of poor pain control. Previous studies used lornoxicam for reduction of postoperative opioid consumption but none of them had studied the intra-operative use of lornoxicam. So, we selected certain type of surgical procedures which might be suitable to use lornoxicam as a sole intra-operative analgesia. The adjunctive use of acetaminophen may have additive analgesic efficacy to lornoxicam because of its intrinsic opioid-sparing activity. Measurement of serum catecholamine would have been useful. These could be considered as a limitation for the present study. Conclusion: Intravenous 16 mg lornoxicam with the present study design was comparable to 100 µg fentanyl as intra-operative analgesia but more effective than fentanyl in preventing early postoperative pain in mild to moderate ENT surgical procedures. Intravenous lornoxicam 8 mg was not satisfactory as a sole intra-operative analgesia. The overall incidence of adverse effects of lornoxicam was lower than that of fentanyl.
Fig 6: Changes in VAS in PACU Discussion: The use of an opioid, even a short acting one can be associated with adverse effects, which may not be acceptable for patients scheduled for day case surgery. For this reason, it was suggested to substitute an opioid with a non-opioid analgesic for postoperative pain control. The use of a NSAID is associated with adverse effects [1]. Lornoxicam has been successfully used in the prevention and treatment of postoperative pain. It has been shown to be as effective as morphine [4], meperidine [5] and tramadol [6]. To the best of our knowledge, this is the first study to compare the perioperative analgesic efficacy of lornoxicam to fentanyl in patients undergoing day case ENT surgery. We gave Lornoxicam half an hour before induction of anesthesia as the time taken to reach peak plasma concentration (Tmax) was determined to be 0.5 h [7]. During the operative procedure, HR and MAP were significantly higher in group L8 compared to group F and L16. While in PACU, patients in groups F and L8 had higher HR, MAP and VAS score in the early postoperative period compared to patients in group L16. This may be due to inadequate analgesic effect of L8 and the shorter plasma half life of fentanyl compared to L16. The analgesic efficacy of L16 might be attributable to inhibition of cyclo-oxygenase (COX1) and (COX2) activity [2], release of endogenous dynorphin and β-endorphin [5], decrease in peripheral and central prostaglandin production [8] as well as exertion of some of its analgesic activity via the central nervous system [9]. Lornoxicam has a more potent anti-inflammatory and analgesic effect than other oxicams as well as a shorter half life, which decreases the incidence of side effects of drugs with long plasma half life [10]. Arslan and colleagues reported decreased opioid need, PONV and postoperative pain scores when 16 mg of lornoxicam was administered after thyroidectomy [11]. While Xuerong and colleaguessuggested that the increase of postoperative morphine requirements induced by intra-operative administration of fentanyl could be prevented by ketamine or lornoxicam [12]. Rawal reported that NSAIDs are effective as the sole analgesic in a high proportion of cases of mild to moderate pain and it is more convenient to give these drugs by the IV route rather than by IM or rectal administration [13]. The analysis of pain intensity differences was complicated by the fact that many patients postoperatively were asleep at the time their pain assessments were due which may be attributed to effect of opioid and anesthetic medications used. To minimize any missing data we used time to the first dose rescue analgesia (based on changes in hemodynamic data) to evaluate pain intensity differences from baseline. L16 was well tolerated in this study, and was associated with a significantly lower incidence of adverse events than F and L8 which could be due to the opioid side effects in both groups. Norholt and colleaguessupported our results as they reported that, in terms of common acute adverse events, lornoxicam appeared to possess a higher benefit/risk ratio compared with morphine [4]. Zuurmond et al reported that, there is good evidence that avoidance of opioid virtually abolishes the PONV that preclude oral intake of fluids after surgery [14]. In our study, nausea developed in 25.7% of patients in group F, 20% in group L8 but only 8.6% in group L16 who received the least rescue opioid analgesia. Regarding bleeding abnormalities, Hodsman et al reported extensive bleeding required reoperation on two diclofenac group patients submitted to abdominoperitoneal resection of the rectum [15]. In our study no abnormal bleeding was reported by ENT surgeons in any of the study patients. In agreement with our results, Ilias et al[16], Trampitsch et al[17] and Karaman et al [18] used lornoxicam and they did not detect problems with surgical bleeding, bleeding time, blood transfusion requirement or postoperative bleeding. Stroissnig et al reported that overall, in healthy adult volunteers, oral doses of lornoxicam up to 70 mg have been well tolerated, and there have been no effects on vital signs, urine analysis parameters or clinical serum biochemistry [19]. In our study, none of the patients receiving study drugs experienced severe gastric discomfort, needed rescue antiemetic medication or required admission because of poor pain control. Previous studies used lornoxicam for reduction of postoperative opioid consumption but none of them had studied the intra-operative use of lornoxicam. So, we selected certain type of surgical procedures which might be suitable to use lornoxicam as a sole intra-operative analgesia. The adjunctive use of acetaminophen may have additive analgesic efficacy to lornoxicam because of its intrinsic opioid-sparing activity. Measurement of serum catecholamine would have been useful. These could be considered as a limitation for the present study. Conclusion: Intravenous 16 mg lornoxicam with the present study design was comparable to 100 µg fentanyl as intra-operative analgesia but more effective than fentanyl in preventing early postoperative pain in mild to moderate ENT surgical procedures. Intravenous lornoxicam 8 mg was not satisfactory as a sole intra-operative analgesia. The overall incidence of adverse effects of lornoxicam was lower than that of fentanyl.
|
Competing Interests None Declared Author Details <p>MOHAMED DAABISS, M.D.Department of Anesthesia, Riyadh Military Hospital, Riyadh, Saudi Arabia. MEDHAT AL-SHERBINY,Department of Anesthesia, Riyadh Military Hospital, Riyadh, Saudi Arabia. RASHID AL-OTAIBI, RIMA AL-NIMAR, Department of Anesthesia, Department of Pharmacy, Riyadh Military Hospital, Riyadh, Saudi Arabia. RIMA AL-NIMAR, Department of Pharmacy, Riyadh Military Hospital, Riyadh, Saudi Arabia</p> CORRESPONDENCE: Dr. DAABISS M. Department of Anaesthesia, Riyadh Military Hospital. Personal Mail Box: D186, P.O.Box 7897, Riyadh 11159, Saudi Arabia Email: madaabiss@yahoo.com |
References
1. Nuutinen LS, Laitinen JO, Salomaki TE. A risk-benefit appraisal of injectable NSAIDs in the management of postoperative pain. Drug Safety 1993; 9:380-93.2. McCorrmack K. The evolving NSAID: focus on Lornoxicam. Pain Rev 1999; 6 (4), 262-78.3. Pruss TP, Stoissnig H, Radhofer-Welte S, et al. Overview of the pharmacological properties, pharmacokinetics and animal safety assessment of lornoxicam. Postgrad Med J 1990; 66 (suppl4):435- 50.4. Norholt ES., Pedersen S, Larsen U. Pain control after dental surgery: a double blind, randomised trial of lornoxicam versus morphine. Pain 1996; 67: 335-43.5. Balanika M., Tsitsika M., Wilczynski W. The use of lornoxicam-mepridine combination for postoperative analgesia. Eur J Anaesthiol 2000; 17, 771-8.6. Staunstrup H, Ovesen J, Larsen T. Efficacy and tolerability of lornoxicam versus tramadol in postoperative pain. J Clin Pharmacol 1999; 39: 834-41.7. Ankier SI, Brimelow AE, Crome P et al. Chlortenoxicam pharmacokinetics in young and elderly human volunteers. Postgrad Med J 1988; 64: 752–754.8. Hitzenberger G, Radhofer-Welte S, Takacs F, et al. pharmacokinetics of lornoxicam in man. Postgrad Med J. 1990; 66(Suppl 4): S22-7.9. Buritova J, Besson JM. Dose-related anti-inflammatory analgesic effects of lornoxicam: a spinal c-Fos protein study in the rat. Inflamm Res 1998; 47 (1), 18-25.10. Radhofer-Welte S, Rabasseda X: Lornoxicam, a new potent NSAID with an improved tolerability profile. Drug Today 2000; 36: 55- 76.11. Arslan M, Tuncer B, Babacan A, et al. Postoperative analgesic effects of lornoxicam after thyroidectomy: A placebo controlled randomized study. Experiment clin studies 2006; 18 (2), 27-33.12. Xuerong Yu, Yuguang H, Xia J, et al. Ketamine and Lornoxicam for Preventing a Fentanyl-Induced Increase in Postoperative Morphine Requirement. Anesth Analg 2008; 107:2032-7.13. Rawal N. Analgesia for day-case surgery. Br J Anaesth 2001, 87(1): 73-87.14. Zuurmond WWA, Van Leeuwen L. Alfentanil vs. isoflurane for out-patient arthroscopy. Acta Anaesthesiol Scand 1986; 30: 329–31.15. Hodsman NB, Burns J, Blyth A, et al. The morphine sparing effects of diclofenac sodium following abdominal surgery. Anesthesia 1987; 42: 1005-8.16. Ilias W, Jansen M. Pain control after hysterectomy: An observerblind, randomised trial of lornoxicam versus tramadol. BJCP 1996; 50: 197-202.17. Trampitsch E, Pipam W, Moertl M, et al. Preemptive randomized double-blind study with lornoxicam in gynecological surgery. Schemerz 2003; 17: 4-10.18. Karaman Y, Kebapci, Gurkan A. The preemptive analgesic effect of lornoxicam in patients undergoing major abdominal surgery: A randomized controlled study. Int J Surg 2008; 6(3): 193-6.19. Stroissnig H, Frenzel W. Lornoxicam. A novel highly potent anti-inflammatory and analgesic agent. Clinical Investigator's Brochure.6th ed. Vienna Hafslund Nycomed Pharma AG, 1992.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




