A rarity that can lead to a casualty - A retrospective study of 12 cases of Dermatomyositis
Matilda Naesström, Monika Kakol, Victoria Kamkar, Wioletta Baranska-Rybak, Malgorzata Sokolowska-Wojdylo, Marta Stawczyk and Roman Nowicki
Cite this article as: BJMP 2015;8(3):a822
|
|
Abstract Aims: Lesions of the skin are omnipresent in Internal Medicine practice. The varying etiopathology when facing multiple system involvement may pose a challenge when it comes to diagnostics and management, especially when faced with less common skin diseases. Dermatomyositis is a rare skin disorder that manifests on the skin and in muscle; it also comes with a higher risk of comorbid cancers. Therefor we present the cases of dermatomyositis diagnosed at our departmet during the last 17 years, with the specific attention to ocurrance of oncological processes. Abbreviations: DM - Dermatomyositis, EMG - Electromyography, ANA - Antinuclear antibodies, CK - Creatine kinase, LDH - Lactate dehydrogenase, AST- aspartate transaminase, ALT - Alanine transaminase, CT - Computer tomography |
Introduction
Dermatomyositis (DM) is a rare autoimmune process with not yet fully understood aetiology. It is characterised by a combination of striated muscle inflammation and cutaneous changes. The pathogenesis of the cutaneous manifestations of DM is not well understood either. DM occurs in all age groups. Therefore, two clinical subgroups of DM are described: adult and juvenile. The adult form is predominant among female patients with a clinical presentation which includes a Heliotrope rash (Fig. 1), Gottron’s papules (Fig. 2), nail fold telangiectasia and other various cutaneous manifestations in association with inflammatory myopathy.1 In addition to the previous mentioned symptoms, juvenile patients also commonly suffer from ulcerative skin and recurrent abdominal pain due to vasculitis. An increased occurrence of oncological processes in combination with adult DM has been observed with a slight predominance for the female gender.2 These patients carry a higher risk for comorbid cancers. The most common ones include malignant processes of the ovary, lung, pancreas, stomach, urinary bladder and haematopoietic system.3 The significance of these observations is that the development of DM should raise suspicion with regard to a possible parallel oncological process.
Figure 1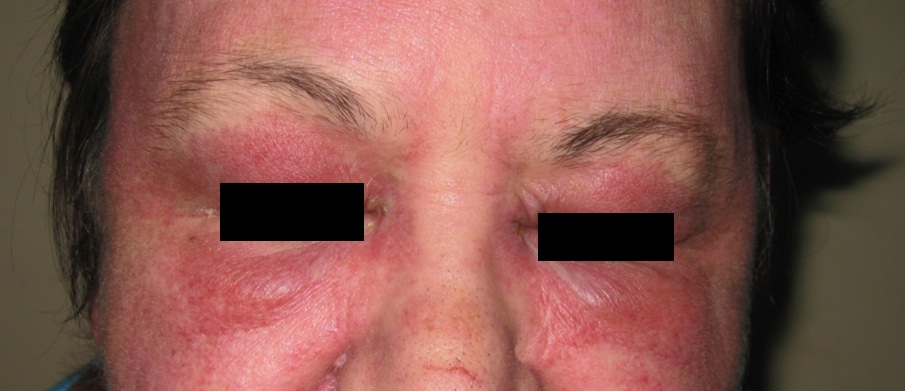
Figure 2
Materials and Methods
A retrospective consecutive case series was performed on a group of 12 patients that were hospitalised at the Department of Dermatology, Venereology and Allergology at the Medical University of Gdansk between 1996 and 2013. The diagnostic criteria for DM included: hallmark cutaneous lesions of DM, clinically significant muscle weakness evaluated by electromyography (EMG), indicative laboratory findings - muscle enzymes, muscle biopsy, autoantibodies. All 12 cases had muscle biopsy, serum studies and EMG performed. The retrospective study analysed the age and sex of the patients, course of the disease, accompanying diseases, clinical picture and treatment. The patients with malignancies were analysed by the primary organs of origin, and the period between the diagnosis of DM and that of malignancy (Table 1).
Table 1. Patient characteristics
| No. | Sex | Previous medical history | Age of onset of DM | Clinical picture | Diagnostics | Treatment | Malignancy and age at diagnosis |
| 1 | F | Chronic eosinophilic leukaemia | 54 | Muscle weakness of shoulder and hip area, facial oedema and erythema, palmar erythema | CK 2550, ANA Hep-2 1:640, LDH 901, AST 69, ALT 143, X-ray = N, USG = N, EMG = N | Azathioprine, Prednisone | Stage IIA ovarian cancer at 55 |
| 2 | F | Peptic ulcer disease | 66 | Facial erythema, Gottron’s papules on the hands, muscular weakness creating difficulty in movement, weight loss, decreased appetite | ANA Hep-2 1:1280, CT = N, EMG = N | Glucocortico- steroids | Small cell carcinoma at 66 |
| 3 | F | None | 23 | Muscular weakness of shoulder and hip area; difficulty in standing up and walking up stairs, Gottron’s papules, Heliotrope rash, upper chest erythema | ANA Hep-2 1: 2580, CPK 12022; AST 595, ALT 210, CK-MB 534; Jo 1 = N, Mi = N | Azathioprine, Prednisone Methotrexate | None |
| 4 | F | Chronic obstructive pulmonary disease | 42 | Muscular weakness of shoulder and hip area, facial oedema and erythema | Cyclo- phosphamide, Methyl- prednisolone | Stomach tumour at 43 | |
| 5 | F | None | 22 | Muscle weakness, painful extremities, facial oedema and erythema | ANA Hep-2 = N, CT = N | Cyclo- phosphamide, Prednisone | None |
| 6 | F | None | 42 | Muscle weakness, paraesthesia of hands, facial oedema and erythema | ANA Hep-2 1:640 | Cyclo- phosphamide, Prednisone | None |
| 7 | F | Hypertension, diabetes type II, osteopenia, leiomyoma. | 65 | Muscle weakness of shoulder and hip area, facial oedema and erythema | ANA Hep-2 1:1280, LDH 650 | Cyclo- phosphamide, Prednisone | None |
| 8 | F | Hyper-thyroiditis | 46 | Muscle weakness; difficulty in moving, facial oedema and erythema | ANA Hep-2 1:160 | Cyclosporine A, Prednisone | None |
| 9 | F | Autoimmune hepatic disease, leiomyoma. | 45 | Muscular weakness of shoulder and hip area, facial oedema and erythema | ANA Hep-2 1:2560, CK 3700, Mi-2 = P | Azathioprine, Methyl-prednisolone | None |
| 10 | F | Hypertension, diabetes type 2, hypo-thyroidism, ovarian cysts | 57 | Muscle weakness of shoulder and hip area, facial oedema and erythema, upper chest erythema, Gottron’s papules, Gottron’s papules, fatigue, dysphagia | ANA Hep-2 1: 640, CK 747, LDH 363, AST 78, Ro52 = P, Mi 2 = N, Jo 1 = N, PM/Scl = N, CT= two pulmonary lesions that were biopsied and diagnosed as pneumoconiosis | Prednisone, Methotrexate | Cervical Carcinoma at 51, Breast Cancer at 57, Pulmonary Metastasis at 58 |
| 11 | F | hypertension | 80 | Muscle weakness, Heliotrope rash
. |
ANA Hep-2 = P; Mi = N, CK 171.5, AST 45.22 | Azathioprine, Prednisone | None |
| 12 | F | hypertension, diabetes Type 2, hypo-thyroidism | 42 | Muscle weakness, Heliotrope rash, upper chest erythema | ANA Hep-2 1:320, Jo 1(-), M(-), CT=N | Cyclosporin A, Methyl- prednisolone, Methotrexate | None |
No. = number (patient), DM = dermatomyositis, F = female, M = male, CK = creatine phosphokinase, ANA = antinuclear antibodies, LDH = lactate dehydrogenase, AST = aspartate transaminase, ALT = alanine transaminase, N = negative, P = positive, USG = ultrasonography, EMG = electromyography, CT = computerised tomography
Limitations
The small sample size is a significant limitation in this retrospective analysis. DM is a rare disease with a prevalence of 1:1000. Increasing sample size, by combining cases from multiple institutions, and implementing control would further strengthen the presented material.
Results
The average age of onset of the disease was 48 years. All 12 subjects were female. Previous medical history included chronic eosinophilic leukaemia, diabetes mellitus type II, hypertension, leiomyomas, hypo- and hyper- thyroid disease, chronic obstructive pulmonary disease, peptic ulcer disease, autoimmune hepatitis and osteopenia. The two most common are diabetes mellitus type II and hypertension. The clinical picture of each case was similar in that all of the patients presented with some form of muscle weakness. In addition, typical features of DM with Gottron’s papules, periorbital oedema, facial oedema and erythema were noted in five patients. Antinuclear Antibodies (ANA) Hep-2 of values >1:160 were identified in nine patients. Additional laboratory markers such as creatine kinase (CK), lactate dehydrogenase (LDH), aspartate transaminase (AST) and alanine transaminase (ALT) were elevated in five patients. Two patients had muscle biopsies performed. The immunohistopathology picture consisted of Immunglobulin G (IgG), fibrinogen, C1q, and C3 deposition around the perimysium and granular deposits of Immunoglobulin M (IgM) in the dermal epidermal junction. Of the 12 patients, four had neoplasms in addition to the diagnosed DM. The primary cancers were originating from the cervix, breast, stomach and ovary. Of these four patients, all had the diagnosis of DM prior to the diagnosis of a malignancy.
Discussion
The diagnosis of DM is made by combining the clinical picture with the results of various laboratory findings: skin and muscle biopsies, EMG, serum enzymes and ANAs.
The clinical picture varies. The typical dermatological presentation consists of a erythematous and oedematous periorbital rash - the Heliotrope rash (Fig. 1). Symmetrical redness and flaking can be observed on the elbows and dorsal sides of the phalanges, especially over the distal metacarpal joints - Gottron’s papules (Fig. 2). Erythematous lesions can also be found on other locations such as the face, upper chest and knees.4 The dermatitis heals with atrophy, leaving behind areas that resemble radiation-damaged skin. The striated muscle inflammation most often involves the shoulder and hip area, leading to muscle weakness and atrophy. The intercostal muscles and the diaphragm may be involved causing alarm with regards to respiratory compromise. Dysphagia can be present due to inflammation of the smooth and skeletal muscles of the oesophagus. These inflammatory processes often lead to muscle calcification.5 The sum of all these changes clinically is seen most often as weakness, weight loss and subfebrile temperatures. All patients in our study had co-existing muscle and cutaneous symptoms, with variation in severity and localisation. Five patients had the classical picture of shoulder and hip area weakness. The rest of the patients had a more general muscle weakness. Two patients had atypical complaints of hand paraesthesia and extremity pain respectively.
Subtypes of DM exist for the purpose of epidemiological research and sometimes prognosis. They are categorised by the clinical presentation and presence or absence of specific laboratory findings. These subtypes are as follows: Classic DM, Amyopathic DM, Hypo-amyopathic DM and Clinically Amyopathic DM. These subtypes have little impact on routine diagnosis. Common laboratory findings in DM are enzymatic elevation of CK, AST, ALT and LDH; these mainly reflect the muscle involvement. Amyopathic DM lacks both abnormal muscle enzymes and weakness.6 Enzymatic elevation may sometimes precede the clinical symptoms of muscle involvement. Hence, an enzymatic raise in a patient with a history of DM, should raise suspicion of recurrence. Positive ANA findings are frequent in DM but not necessary for diagnosis. More myositis-specific antibodies include anti-Mi 2 and anti-Jo 1. A typical histopathological examination shows: myofiber necrosis, perifascicular atrophy, patchy endomysial infiltrate of lymphocytes and occasionally the capillaries may contain membrane attack complexes.7
Cutaneous changes and muscular complaints can correspond to: 1. Systemic scleroderma which often has a positive ANA; 2. Trichinosis, in which periorbital swelling and myositis occurs, but there is a prominent eosinophilia and a history of consuming undercooked swine or bear meat; 3. Psoriasis with joint involvement which may give a clinically similar picture to DM. However, the skin changes in psoriasis have a more flaking pattern. In doubtful cases, a skin and muscle biopsy together with an electromyography will set the diagnoses apart. A facial rash may also be observed in systemic lupus erythematosus together with nail fold telangiectasia. They are usually distinguished by a clinical picture with more organ system involvement in systemic lupus and by serological studies. A drug-induced picture of DM exists and is particularly associated with statins and hydroxyurea.8
It is estimated that around 25% of DM cases are associated with a neoplastic process that can occur prior, during or after the episode of DM. The risk of developing a malignancy is highest in the first year of DM and remains elevated for years after diagnosis. 9, 10, 11 This was the case with patient number 1, 2 and 4 in our study, where the malignant process appeared in the first year following onset of DM. Risk factors seen in DM patients include male gender, advanced age and symptoms of dysphagia.12 The age range of the four patients in our study with malignancy was between 43 and 66. Symptoms that clinically raised suspicion of a malignant process included weight loss, lack of appetite and dysphagia. All neoplasms were discovered within one year after the diagnosis of DM was made. One patient had a previous history of cervical cancer, six years prior to the onset of DM.
The most common neoplasms seen in patients with DM vary in the world. In Europe the malignancies are located mainly in the ovaries, lungs, and stomach. The cancer types associated with the DM correlate with common cancers seen in the same area. For instance, in Asia, nasopharyngeal carcinoma (which is a rare malignancy in Europe) is a frequent occurrence in DM.1, 3 The location of neoplasms seen in our study varied from gastric, breast, ovary and pulmonary. The screening in regards to malignancies in patients with DM is individualised and should be based on risk factors such as previous malignancies, alarming symptoms such as weight loss or dysphagia, or abnormal findings on physical exam. This was the case with patient number 10 in our study who had a previous history of cancer, and patient number 2 who had symptoms of weight loss and decreased appetite. Initial screening was negative for patient number 1 and 2, where the malignancy developed first after the onset of DM. Age-appropriate screening with mammography, faecal-occult blood test and Papanicolaou smear should be considered. Additional investigations with chest films, computerised tomography (CT) scanning of chest, abdomen or pelvis; colonoscopy, cancer antigens; and gynaecological ultrasonography should be done when indicated.
The main objective of treatment in DM is to improve muscle strength and obtain remission, or at least clinical stabilisation. No specific protocol exists with regard to treatment of DM. Treatment is individualised and adapted to the specific condition of the patient. High-dose corticosteroids are the basis of treatment. However, randomised placebo clinical trials failed to show their efficacy. Clinical efficacy of corticosteroid therapy demonstrates itself and hence is the initial treatment of choice. Doses start at around 1 mg/kg/day depending on the corticosteroid of preference. This dosing is maintained for approximately two months until clinical regression is achieved, followed by approximately 10 mg decrease in dose for the coming three months. A maintenance dose of approximately 5-10 mg should be achieved. The exact parameters are patient-specific. In the case of a severe flare of dermatomyositis, 1 g per day for three days of methylprednisolone intravenous pulses can be administered. The systemic effects of long term therapy with corticosteroids have to be kept in mind. Hence, yearly dual-energy X-ray absorptiometry bone scans can be administered to monitor the development of osteopenia.
Further treatment options are offered in situations where the initial disease presentation is severe, involves internal organs, if relapse occurs during steroid dose reduction, and steroid side-effects. It has been proposed that combination therapy is a better method of approach due to lower reported relapse rates and lower need to use high-dose corticosteroids. Methotrexate is second-line therapy when steroids fail alone. Methotrexate is used with a maximum dose of 25 mg per week plus folate supplementation. The limitations of Methotrexate are immunosuppression and pulmonary fibrosis. Methotrexate is considered preferable to Azathioprine because the latter has a longer onset of efficacy. Azathioprine is administered at doses ranging from 1.5 - 3 mg/kg/day and has a side-effect profile is similar to that of other immunosuppressants. Cyclosporin A is a T-cell cytokine moderator that has a similar efficacy profile to Methotrexate. Side-effects include renal impairment, gingival hyperplasia, and hypertrichosis. Dosing of Cyclosporin A ranges from 2 - 3 mg/kg/day.
An expensive but effective and rather low side-effect alternative is intravenous immunoglobulins. The dosage of this medication has not been officially established in the treatment of DM, but options are: 2 g/kg given either in 1 g/kg/day for two days every four weeks; or 0.4 mg/kg/day for five days initially, and then for three days monthly for three to six months. Other alternatives include Mycophenolate Mofetil, Cyclophosphamide, Chlorambucil, Fludarabine, Eculizumab, Rituximab.9 Further options might be treatment targeted toward malignancy when associated with DM. This was observed in our patient number 10, where full remission of DM was obtained first after lobectomy and chemotherapy for the mammary carcinoma.
Conclusion
DM mainly affects women and all 12 cases presented in our study were female. One third of our cases had malignancies associated with their course of DM. We conclude that it is reasonable to screen these patients, especially in those with already established cancer risk factor. Age-appropriate screening and beyond is indicated by high risk factors or clinical presentation. High suspicion should be raised in patients with a previous history of oncological treatment since DM can be the first clinical sign of cancer recurrence.
|
Competing Interests None declared Author Details MATILDA NAESSTRÖM, M.D., Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. MONIKA KAKOL, M.D. , Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. VICTORIA KAMKAR, M.D. , Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. WIOLETTA BARANSKA-RYBAK, M.D PhD, Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. MALGORZATA SOKOLOWSKA-WOJDYLO, M.D. , Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. MARTA STAWCZYK, M.D. , Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. Prof ROMAN NOWICKI, M.D. , Department of Clinical Dermatology, Venerolgy and Allergology, Medical University of Gdansk, Poland. CORRESPONDENCE: MATILDA NAESSTRÖM, Department of clinical Dermatology, Venerology and Allergology Medical University of Gdansk, M. Skłodowskiej-Curie 80-210, Gdansk, Poland. Email: matilda.naesstrom@hotmail.com |
References
- Liu WC, Ho M, Koh WP et al.. An 11-year review of dermatomyositis in Asian patients. Ann Acad Med Singapore 2010;39:843-847.
- Ohashi M, Shu E, Tokozumi M Fujioka K et al. Anti-p155/140 antibody-positive dermatomyositis with metastases origins from an unknown site. Acta Derm Venereol 2011;91:84-85.
- Chen YJ, Wu CY, Huang YL et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther2010;12:R70.
- Callen JP. Dermatomyositis. Lancet. Jan 1 2000;355(9197):53-7
- Wananukul S, Pongprasit P, Wattanakrai P. Calcinosis cutis presenting years before other clinical manifestations of juvenile dermatomyositis: report of two cases. Australas J Dermatol. 1997 Nov;38(4):202-5.
- Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermato myopathies spectrum of clinical illness?. J Am Acad Dermatol. Apr 2002;46(4):626-36
- Smith ES, Hallman JR, DeLuca AM et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. Feb 2009;31(1):61-7.
- Seidler AM, Gottlieb AB. Dermatomyositis induced by drug therapy: a review of case reports. J Am Acad Dermatol. 2008 Nov;59(5):872-80.
- Callen JP, Hyla JF, Bole GG Jr et al. The relationship of dermatomyositis and polymyositis to internal malignancy. Arch Dermatol. Mar 1980;116(3):295-8.
- Buchbinder R, Forbes A, Hall S et al.. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. Jun 19 2001;134(12):1087-95.
- Chow WH, Gridley G, Mellemkjaer L et al.Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. Jan 1995;6(1):9-13.
- Chen D, Yuan S, Wu X et al. Incidence and predictive factors for malignancies with dermatomyositis: a cohort from southern China. Clin Exp Rheumatol. Jul 28 2014
- Cordeiro AC, Isenberg DA. Treatment of inflammatory myopathies. Postgrad Med J 2006;82:417-424

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




