Sudden Death in a Patient with Left Atrial Myxoma: Report of two cases and review of literature
Kalgi Modi, Prasanna Venkatesh, Sujata Agnani, Tanya Rowland and Pratap Reddy
Cite this article as: BJMP 2010;3(2):318
|
|
Abstract Sudden death is known to occur in patients with primary cardiac tumours; however it is rare and is estimated to constitute 0.005% of all sudden deaths. We report here two cases of sudden death that occurred in patients with left atrial myxoma. We also present a brief review of available literature on this subject.
|
Case 1
A 55 year old white male with a history of hypertension, hyperlipidemia, smoking and transient ischaemic attacks was admitted to the hospital with worsening dyspneoa on exertion over a period of 6 weeks. He also reported significant weight loss, loss of appetite and fatigue over several weeks. Physical examination revealed tachycardia, and moderate respiratory distress with prominent jugular venous distention. Cardiac auscultation revealed normal S1 and loud P2. Also heard were an early diastolic heart sound ( tumour plop) and a mid-diastolic murmur at the apex. An ECG revealed evidence of left ventricular hypertrophy with repolarization abnormalities. A transthoracic echocardiogram (Figures 1 and 2) revealed a large, pedunculated, mobile left atrial mass measuring 3x4 cm, impinging on the mitral orifice with a mean gradient across the mitral valve of 15 mm Hg. Left ventricular systolic function was normal.

Figure 1: Parasternal long axis echocardiograph of the left atrial myxoma prolapsing into the mitral valve during diastole.
A diagnosis of probable left atrial myxoma was made. The patient had four episodes of syncope within 24 hrs, the first at 3: 53 am after returning from the bathroom, subsequently leading to cardiac arrest at 14:20 pm.
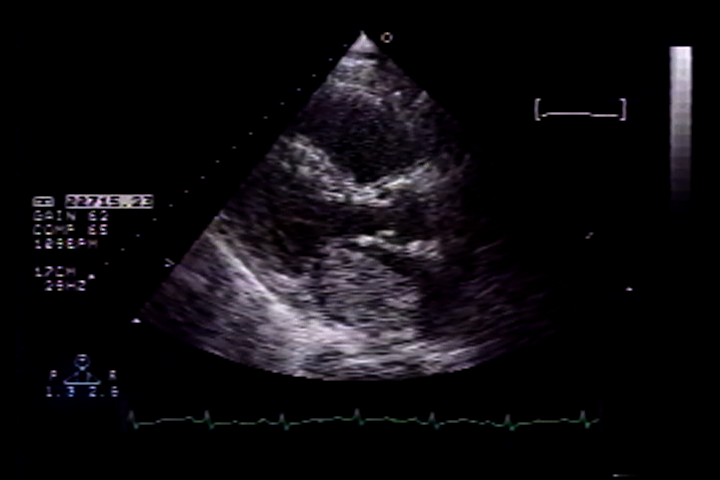
Figure 2: parasternal long axis echocardiograph showing the large left atrial myxoma during systole.
He was intubated and initiated on vasopressors. An emergent Left heart catheterization was performed prior to a referral for surgical excision, which revealed triple vessel coronary artery disease. During cardiac catheterization the patient became more hypotensive requiring an intra-aortic balloon pump. While arrangements were made for a referral for surgery, the patient’s clinical condition deteriorated rapidly and he went into pulseless electrical activity at 18:54 pm and could not be resuscitated. The patient’s death was presumably due to persistent intracardiac obstruction. On autopsy, the left atrial mass was identified as a haemorrhagic left atrial myxoma, 5x4x3.5cm in size attached by a stalk to an inter-atrial septum. Multiple organizsing thrombi were present in the 1tumour. Histology showed abundant ground substance with stellate myxoma cells and haemosiderin-laden macrophages (Figures 3 and 4). The cause of death was attributed to valvular “ball-valve” obstruction.
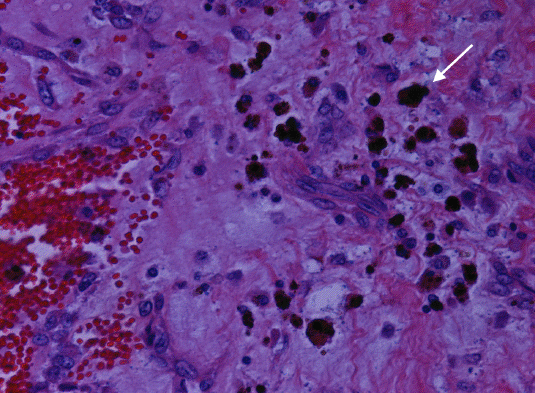
Figure 3: Histopathology of left atrial myxoma showing spindle shaped myxoma cells (white arrow) in a myxoid matrix (black arrow) and blood vessels (top arrow) (H & E 40X)
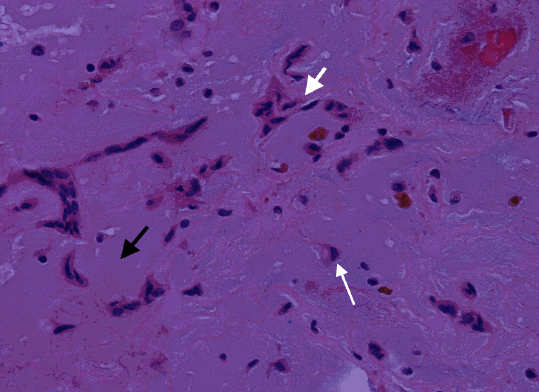
Figure 4: Histopathology of left atrial myxoma showing vascular spaces filled with relatively fresh blood and evidence of old bleeding (hemosiderin) suggesting repeated episodes of hemorrhage within the myxoma (H & E 4X)
Case 2
A 57 year old African American female presented with recurrent syncopal episodes and dyspnea on exertion, orthopnea, leg swelling, abdominal distention, loss of appetite and fatigue for the preceding nine months. Physical examination revealed jugular venous distention, a displaced apical cardiac impulse, a parasternal heave, and a loud S2. Also detected were a pan-systolic murmur at the lower left sternal border, an early diastolic heart sound with a mid diastolic murmur at the apex, bibasilar crackles, ascites, and oedema up to the thighs.
Significant laboratory values were a total bilirubin of 1.6 mg/dl, and B- Type Natriuretic Peptide of 1323 pg/ml. A chest x-ray revealed an enlarged cardiac silhouette, right lung atelectasis and effusion. An ECG revealed left atrial and right ventricular enlargement.
The patient was admitted with the diagnosis of new onset congestive heart failure and was treated with intravenous lasix, and fosinopril. A 2-D Echocardiogram revealed a large mass suggestive of myxoma in the left atrium measuring 4.5 x 7.5 cm, occupying the entire left atrium protruding through the mitral valve into the left ventricle (Figure 5) .

Figure 5: Apical four chamber echocardiograph of the left atrial myxoma prolapsing into the mitral valve during diastole.
This mass was obstructing flow with a mean trans mitral gradient of 17 mm Hg, with a reduced stroke volume and severe pulmonary hypertension with an estimated Right Ventricular systolic pressure of 120 mm hg. A presumptive diagnosis of left atrial myxoma was made and the patient was scheduled for its surgical removal the following morning. The patient was transferred to the intensive care unit for closer monitoring; and fosinopril and lasix were discontinued. At about 22:30 hours that night patient was noted to be hypotensive with systolic blood pressure of around 80mm Hg. The patient was treated with normal saline and concentrated albumin. She then developed acute respiratory distress at 23:00 hours requiring intubation and ventilator support. Intravenous dobutamine, dopamine and later norepinephrine were added for continued hypotension. The patient went into pulseless electrical activity, she was successfully coded with a return of her pulse but continued to be hypotensive. Cardiothoracic surgery decided not to take the patient for emergency surgery due to her unstable haemodynamic condition. The patient’s family was notified of the poor prognosis and the decision was made not to resuscitate her if her condition deteriorated further. The patient ultimately became bradycardic and went into asystole at 5: 30 am. An autopsy was not performed. The cause of death was attributed to large left atrial myxoma causing valvular obstruction and cardiovascular collapse.
Discussion
These two cases illustrate an uncommon, malignant course of a left atrial myxoma with rapid progression of symptoms which proved fatal. The most common primary tumour of the heart is myxoma accounting for 40-50% of primary cardiac tumours(2,3) .Nearly 90% of myxomas occur in the left atrium(3) .In over 50% of patients, left atrial myxoma causes symptoms of mitral stenosis or obstruction. Systemic embolic phenomena are known to occur in 30-40% of patients(3) .
Table 1. Summary of 17 published cases of sudden cardiac death associated with cardiac myxoma in adults (1950-2008)
|
Author/Reference
|
Year
|
No
|
Age
|
Gender
|
Symptoms
|
Interval Between Symptoms To Scd
|
Size Of Myxoma In Cm
|
Autopsy
|
|
Vassiliadis (8)
|
1997
|
1
|
17
|
M
|
Dizziness
|
3 months
|
6
|
yes
|
|
McAllister (10)
|
1978
|
5
|
40 to60
|
NA
|
NA
|
NA
|
5 to 6
|
yes
|
|
Cina (2)
|
1996
|
6
|
Below 40
|
NA
|
Embolic, syncope
|
16.6 months
|
5.7
|
yes
|
|
Puff (9)
|
1986
|
1
|
41
|
M
|
Syncope,
|
months
|
1.5
|
yes
|
|
Puff (9)
|
1986
|
1
|
19
|
F
|
Syncope
|
6 months
|
3
|
yes
|
|
Maruyama (7)
|
1999
|
1
|
20
|
M
|
Dizziness
|
1 day
|
8
|
None, Patient survived SCD; Myxoma resected
|
|
Turkman (6)
|
2007
|
1
|
73
|
M
|
DOE
|
months
|
8
|
yes
|
|
Ito (13)
|
1987
|
1
|
28
|
M
|
Syncope
|
7 days
|
NA
|
yes
|
NA: not available
Constitutional symptoms reported in approximately 20% of patients include myalgia, muscle weakness, athralgia, fever, fatigue, and weight loss. Around 20% of cardiac myxomas are asymptomatic (3) .Severe dizziness/syncope is experienced by approximately 20% of patients due to obstruction of the mitral valve. (4) Of all the symptoms associated with cardiac myxomas, syncope is one of the most ominous prognostic indicators.
Although sudden death is known to occur in patients with primary cardiac tumour it is rare and is estimated to constitute 0.01 to 0.005% of all sudden deaths (1). Association between sudden death and cardiac myxoma has been reported as early as 1953 by Madonia et al (5). A review of the literature on this subject between 1950 to2008 revealed 17 cases of sudden death attributed to cardiac myxoma in adults (1, 6, 7, 8, 9, 10, 13) (Table 1) .
In all patients with unexpected death syncope was a predominant presenting symptom and their age ranged from seventeen to seventy three. The majority of patients with sudden death were men even though the tumour is more common in women. The size of the tumour did not influence clinical presentation and in some reports of sudden cardiac death tumour was as small as 1.5 cm and without previous symptoms (3). Sudden death in myxoma is attributed to either severe acute disturbance in cardiac haemodynamics from cardiac obstruction (ball-valve syndrome) or to coronary embolization from the tumour. The latter is probably responsible for sudden death in patients with very small tumours. In the study of Alverez Sabin et al (11) the initial neurological manifestation was Transient Ischemic Attack (TIA), but in none of the patients’ was a diagnosis of myxoma made because of the initial neurological symptom. Even though cardiac myxomas are a rare cause of TIA and syncope, it is important to consider cardiac myxoma in the differential diagnosis of any patient with a TIA or syncope (11). The patients presented here had a TIA and recurrent syncope placing them at high risk for sudden death.
The timing of surgical excision of myxoma is not clear and it is not unusual for patients to die or experience a major complication while awaiting surgery (2, 12). Intraaortic balloon pump (IABP) use has been described in one case of left atrial myxoma and life-threatening cardiogenic shock with favorable outcome(14) .As illustrated by the cases presented here it is essential that surgery be performed urgently once it has been identified that a patient has a myxoma that is large enough to cause complete intracardiac obstruction.
|
Acknowledgements David Baldwin, Marlissa Curtis, Mike Yates and Baryl Cowthran for images IRB approved Competing Interests None Declared Author Details Kalgi Modi MD, FACC, Prasanna Venkatesh MD, Sujata Agnani MD, Tanya Rowland MD, Pratap Reddy MD, FACC, Department of Medicine, Section of Cardiology, Louisiana State University Health Science Center. CORRESPONDENCE: Kalgi Modi MD, FACC, 1501 Kings Highway Shreveport, LA 71106 Email: kmodi@lsuhsc.edu |
References
1. Cina SJ, Smialek JE, Burke AP, et al . Primary cardiac tumors causing sudden death: a review of the literature. American Journal of Forensic Medicine & Pathology. 1996; 17(4):271-81.
2 Reynen, K, M.D. Cardiac Myxoma NEJM. 1995; 14:1610 -16176.
3. Burke A, Jeudy J, Virmani R, Cardiac Tumors: an update. Heart. 2008; 94: 117-123
4. Kapoor MC, Singh S, Sharma S, Resuscitation of a patient with giant left atrial myxoma after cardiac arrest. Journal of cardiothoracic and vascular anesthesia. 2004; 18:769-771
5. Madonia, PF, Boggiano R, Gubner, R, Ball Valve Syndrome caused by primary cardiac tumor. NY State J Med. 1953; 53:3043-3044
6. Turkmen N. Eren B. Fedakar R. Comunoglu N. An unusual cause of sudden death: cardiac myxoma. Advances in Therapy. 2007; 24(3):529-32 .
7. Maruyama T. Chino C. Kobayashi T. Ohta K. Kono T. Nakano H. A survivor of near sudden death caused by giant left atrial myxoma. Journal of Emergency Medicine. 1999; 17 (6):1003-6.
8. Vassiliadis N. Vassiliadis K. Karkavelas G. Sudden death due to cardiac myxoma. Medicine, Science & the Law. 1997; 37(1):76-8.
9. Puff M. Taff ML. Spitz WU. Eckert WG. Syncope and sudden death caused by mitravalve myxomas. American Journal of Forensic Medicine & Pathology. 1986; 7(1):84-6.
10. McAllister HA, Fenoglio JJ, Tumors of the Cardiovascular System, second series, 1978; fascicle 15: page 5-20
11 J.Alvarez Sabin, M. Lozano, J. Sastre-Garriga, J. Montoyo, M. Murtra, S. Abilleira, Transient Ischemic Attack: A Common Initial Manifestation of Cardiac Myxomas Eur Neurol 2001;45:165-170
12. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine, 7th edition, pp 1745
13. Ito Y, Tsuda R, Hara M. An Autopsy case of sudden death from Left atrial myxoma Nihon Hoigaku Zasshi 1987; 41(4): 369-373.
14. Tanaka K, Sato N, Yamamoto T et al, Measurement of End-tidal Carbon Dioxide in Patients with Cardiogenic Shock Treated Using a Percutaneous Cardiopulmonary Assist System, J Nippon Med Sch 2004; 71(3): 160-166

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




