Ventilator-associated pneumonia: A review of the clinically relevant challenges in diagnosis and prevention
Varun Goel, Savita Gupta and Tarun Goel
Cite this article as: BJMP 2016;9(2):a910
|
|
Abstract Ventilator-associated pneumonia is one of the most commonly encountered nosocomial infections in the intensive care units and is associated with high morbidity and high costs of care. Inspite of extensive studies for decades, a clear diagnostic and prevention strategy is still eluding Ventilator-associated pneumonia. Clinical diagnosis has been criticized to have poor accuracy and reliability. Quantitative cultures obtained by different methods seem to be rather equivalent in its diagnosis. Blood cultures are relatively insensitive to diagnose Ventilator-associated pneumonia. Thus, the Centers for Disease Control and Prevention has introduced a new definition based upon objective and recordable data. New preventive strategies are focused on the improvement of secretions drainage and prevention of bacterial colonization. We performed a literature review to describe the evidence-based Ventilator-associated pneumonia-diagnosis and prevention strategies that have resulted in clinically relevant outcomes. An integrated approach should be followed in diagnosing and preventing Ventilator-associated pneumonia. Keywords: Pneumonia, Nosocomial Infections, Ventilator Associated Pneumonia, ventilator bundle |
INTRODUCTION
Ventilator-associated pneumonia (VAP) is a type of nosocomial pneumonia that occurs in patients who receive mechanical ventilation and is usually acquired in the hospital setting approximately 48–72 hours after mechanical ventilation.1 VAP is one of the most frequent hospital-acquired infections occurring in mechanically ventilated patients and is associated with increased mortality, morbidity, and health-related costs. Several risk factors have been reported to be associated with VAP, including the duration of mechanical ventilation, and the presence of chronic pulmonary disease, sepsis, acute respiratory distress syndrome (ARDS), neurological disease, trauma, prior use of antibiotics, and red cell transfusions.2 VAP occurrence is closely related to intubation and the presence of the endotracheal tube (ETT) itself.
Since there are inadequate objective tools that are utilized to make an assessment of bacterial-induced lung injury in a heterogeneous group of hosts, the diagnosis of VAP is challenging. Around 90% of ICU-acquired pneumonias occur during mechanical ventilation, and 50 % of these ventilator-associated pneumonias begin in the first 4 days after intubation.3 VAP has a cumulative incidence of 10-25% and accounts for approximately 25% of all ICU infections and 50% of its antibiotic prescription, making it the primary focus for risk-reduction strategies.1,4 For all these reasons, early diagnosis and prevention of VAP has held a prominent position on the research agenda of intensive care medicine in the past 25 years, with an ultimate goal of improving patient outcome, preferably by reducing mortality.
The keywords, ‘ventilator-associated pneumonia,’ in PUBMED revealed a total of 3612 titles and 625 review articles within the search limit of 10 years, between 2005 and 2014. Only articles in English were chosen.
PATHOGENESIS
Understanding the pathogenesis of VAP is the first step in the formulation of its appropriate preventive and therapeutic strategies. The initial step in the pathogenesis of VAP is bacterial colonization of the oropharynx and gastric mucosa, followed by translocation of the pathogens to lower respiratory tract. The most common means of acquiring pneumonia is via aspiration which is promoted by supine position and upper airway and nasogastric tube placement.2,5 In a mechanically ventilated patients, aspiration occurs around the outside of the endotracheal tube rather than through the lumen. Secondly, aerobic Gram-negative bacteria presumably reach the lower airway via aspiration of gastric contents or of upper airway secretions. Other means by which VAP can be acquired include aspiration from the stomach or nose and paranasal sinuses. Figure 1 depicts the essential elements favoring colonization of lower respiratory tract with the bacterial pathogens with subsequent development of pneumonia.2,5,6
Figure 1: Pathogenesis of Ventilator-associated pneumonia5
*Gastric alkalinization; prior antimicrobials; ICU stay; intubation; supine position; circuit/airway manipulation and mishandling; device cross-contamination; sedation; diminished cough reflex; and malnutrition predispose to colonization and aspiration. As the duration of ICU stay increases, colonization with MDR Gram-negative pathogens like Pseudomonas and Acinetobacter increases.
†Via contaminated nebulizers/aerosols
Reproduced with permission from the publisher.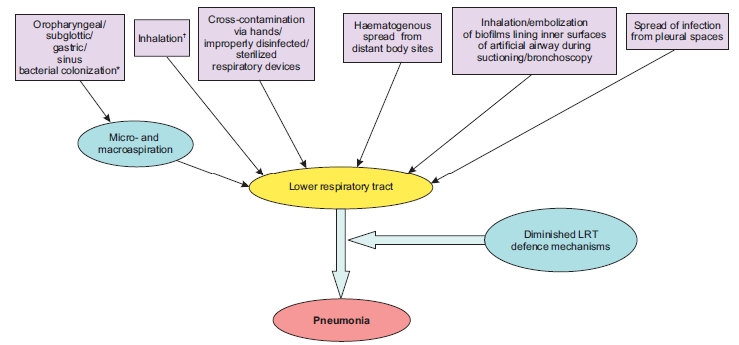
COMMON CAUSES
The specific microbial causes of VAP vary widely depending in epidemiological and clinical factors. Common pathogens include aerobic gram negative bacteria such as Pseudomonas aeruginosa and members of family Enterobacteriaceae, staphylococci, streptococci, and Haemophilus species. Microorganisms like Pseudomonas spp., Acinetobacter spp. and Methicillin-Resistant Staphylococcus aureus occur commonly after prior antibiotic treatment, prolonged hospitalization, mechanical ventilation or when other risk factors are present.6,7
Moreover, deliberated ill patients may have defect in phagocytosis and behave as functionally immunosuppressed even prior to emergence of nosocomial infection as seen by many recent studies.8,9
DIAGNOSIS
Clinical Diagnosis
No gold standard of diagnosis for identifying VAP is there inspite of variety of proposed definitions. VAP has traditionally been diagnosed by clinical criteria of Johanson and colleagues (appearance of new or progressive pulmonary infiltrates, fever, leucocytosis and purulent tracheobronchial secretions), which are non-specific. When findings on histologic analysis and cultures of lung samples obtained immediately after death were used as references, a new and persistent (>48-h) infiltrate on chest radiograph plus two or more of the three criteria (i) fever of >38.3°C, (ii) leukocytosis of >12 × 109/ml, and/or (iii) purulent tracheobronchial secretions had a sensitivity of 69% and a specificity of 75% for establishing the diagnosis of VAP.10
Because of the poor specificity of the clinical diagnosis of VAP and of qualitative evaluation of ETAs, Pugin et al. developed a composite clinical score, called the clinical pulmonary infection score (CPIS), based on six variables: temperature, blood leukocyte count, volume and purulence of tracheal secretions, oxygenation, pulmonary radiography, and semi-quantitative culture of tracheal aspirate. The score varied from 0 to 12. A CPIS of >6 had a sensitivity of 93% and a specificity of 100%.11 Accuracy of CPIS in diagnosis of VAP is debated, despite of its clinical popularity. In one meta-analysis study evaluating the accuracy of CPIS in diagnosing VAP reported pooled estimates for sensitivity and specificity for CPIS as 65 % (95 % CI 61-69 %) and 64 % (95 % CI 60-67 %), respectively.12 The poor accuracy of clinical criteria for diagnosing VAP is due to purulent tracheobronchial secretions in patients receiving prolonged mechanical ventilation which are rarely caused by pneumonia. Moreover, in pneumonia systemic signs such as fever, tachycardia, and leukocytosis are nonspecific; they can be caused by any state that releases the cytokines interleukin-1, interleukin-6, interleukin-8, tumor necrosis factor alpha (TNFα), and gamma interferon.13,14 The weak point of CPIS is probably the inter-individual variability (kappa= 0.16), since a subjective evaluation is required when we are judging the quality of tracheal secretion (purulent/not purulent) and the presence of infiltrate at chest ray.15
Radiologic Diagnosis
Radiographical evidence of pneumonia in ventilated patients is also notoriously inaccurate. In a study of autopsy proven VAP, of the total population, only air bronchograms correlated with pneumonia and no specific roentgenographic sign correlated with pneumonia in patients with adult respiratory distress syndrome. The differential diagnoses of VAP based on radiographical appearance, include adult respiratory distress syndrome, congestive heart failure, atelectasis, pulmonary embolism and neoplastic infiltration.16
Microbiologic Diagnosis
The type of specimen that should be obtained for microbiologic processing as soon as VAP is suspected is another area of importance. The use of quantitative cultures is one of the main issues for any diagnostic laboratory because there is oropharyngeal bacterial contamination of all respiratory secretion samples, despite this is not always undertaken in many hospitals today.16,17
Blood cultures
Blood cultures have limited value because organisms isolated from blood in suspected VAP cases are often from extrapulmonary sites of origin.18 Blood cultures in patients with VAP are clearly useful if there is suspicion of another probable infectious condition, but the isolation of a microorganism in the blood does not confirm that microorganism as the pathogen causing VAP.
Quantitative cultures of airway specimens
Simple qualitative culture of endotracheal aspirates has high percentage of false-positive results due to bacterial colonization of the proximal airways observed in most patients in the ICU.20 Quantitative culture techniques suggest that endotracheal aspirate cultures (QEA) may have an acceptable overall diagnostic accuracy, similar to that with several other, more invasive techniques including BAL, protected BAL (pBAL) ,protected specimen brush (PSB) or tracheobronchial aspirate(TBA).7,19,20 Threshold values often employed for diagnosing pneumonia by quantitative cultures are ≥105 to 106, ≥104, and ≥103 CFU/ml for QEA, bronchoscopic BAL, and PSB, respectively, with ≥105 CFU/ml being the most widely accepted value for QEA.21,22,23 Also, blind aspiration sampling can lead to errors but bronchoscope also carries risks, such as inducing cardiac arrhythmia, hypoxemia, bleeding, pneumothorax, along with greater costs both in terms of time and resources. It is accepted that before administering the first dose of antibiotic or before any change in treatment patient specimens for culture should be taken, so that the results interpreted are valid.24 Lalwani et al., in their study, observed that culture results of a properly collected tracheal aspirate should be taken into consideration along with Centre for Disease Control and Prevention (CDC's) diagnostic criteria to maximize the diagnosis of VAP.25
The recent guidelines of Society for Healthcare Epidemiology of America/ Infectious Diseases Society of America (SHEA/IDSA) recommend Gram staining of endotracheal aspirates. However, the sensitivity (57-95%) and specificity (48-87%) of this technique are highly variable. The role of procalcitonin and other biomarkers for the diagnosis of VAP is yet unsubstantiated.5,26
Since VAP diagnosis founded on radiographic findings of pneumonia, which have intrinsic variability in technique, interpretation, and reporting, and on clinical signs and symptoms- that are subjective- in 2011 a Working Group of the CDC proposed a new approach to surveillance for Ventilator-Associated Events (VAE). Table 1 According to the new CDC definition algorithm, VAP is an Infection-related Ventilator-Associated Complication (IVAC) occurring after 3 days of mechanical ventilation and 2 days before or after the onset of worsening oxygenation, if purulent respiratory secretions with positive cultures or objective signs of respiratory infection have been found.27
Table 1: CDC Algorithm for VAP diagnosis30
| 1= Purulent respiratory secretions AND one of the following: | 2= One of the following (without requirement for purulent respiratory secretions): |
| Positive culture of endotracheal aspirate, ≥ 105 CFU/ml * | Positive pleural fluid culture |
| Positive culture of bronchoalveolar lavage, ≥ 104 CFU/ml* | Positive lung histopathology |
| Positive culture of lung tissue, ≥ 104 CFU/ml* | Positive diagnostic test for Legionella spp. |
| Positive culture of protected specimen brush, ≥ 103 CFU/ml* | Positive diagnostic test on respiratory secretions for influenza virus, respiratory syncytial virus, adenovirus, parainfluenza virus |
|
On or after calendar day 3 of mechanical ventilation and within 2 calendar days before or after the onset of worsening oxygenation, criteria 1 or 2 is met (*or equivalent semi-quantitative result). |
|
Table 2: Practices for which insufficient evidence or no consensus exists about Efficacy8,57
| Rotational or turning therapy | Routine use of turning or rotational therapy, either by ‘kinetic’ therapy or by continuous lateral rotational therapy |
| Systemic antimicrobial agent prophylaxis | Routine administration of systemic antimicrobial agent(s) to prevent pneumonia in those receiving mechanically-assisted ventilation. Changes in the antimicrobial agents class used for empiric therapy |
| Oral chlorhexidine
rinse for oropharyngeal colonization |
Routine use of an oral chlorhexidine rinse for the prevention of healthcare-associated pneumonia in all postoperative or critically ill patients and/or other patients at high risk for pneumonia. |
| Ventilator breathing circuits with HMEs | No recommendation can be made for the preferential use of HMEs to prevent pneumonia in patients receiving mechanically assisted ventilation No recommendation can be made for placing a filter or trap at the distal end of the expiratory-phase tubing of the breathing circuit to collect condensate |
| Suctioning of respiratory tract secretions | No recommendation can be made for the preferential use of either the multiuse closed-system suction catheter or the single-use open-system suction catheter |
| Prevention of aspiration associated with enteral feeding | Small-bore tubes for enteral feeding Enteral feedings continuously or intermittently should be given |
| Patient care with tracheostomy | Daily application of topical antimicrobial agent at the tracheostoma |
| Gloving | Wearing sterile rather than clean gloves when performing endotracheal suctioning |
STRATEGIES FOR VAP PREVENTION
There are multiple recommended measures for prevention of VAP. Practices for which insufficient evidence or no consensus exists about efficacy are summarized in Table 2. Preventive VAP strategies can be grouped into two classes: non-pharmacologic strategies, which are focused on preventing aspiration, and pharmacologic strategies, which are aimed at preventing colonization.
Non-Pharmacologic Strategies
Staff Education in the Intensive Care Unit
Various barriers to adhering to VAP prevention recommendations include disagreement with the reported results of source studies, resource paucity, elevated costs, inconvenience for nurses, fear of potential adverse effects and patient discomfort. There is considerable variability in practice between countries regarding humidification systems, intubation route, endotracheal suction system, kinetic therapy beds, subglottic secretion drainage and body position. For efficient patient care staffing must be sufficient while ensuring that staff is able to comply with essential infection control practices and other prevention strategies.17,28
Hand Hygiene
Microorganisms can be spread easily from patient to patient on the hands of healthcare workers. Moreover, wrist watches, rings, bangles and other jewelry commonly act as reservoirs for organisms, and impede effective hand cleaning. Moreover, healthcare workers compliance to hand hygiene is low, and high workload decreases their compliance.29
Impact of patient position
Patients positioned semi-recumbently 45 degrees have significantly lower incidence of clinically diagnosed VAP compared to patients positioned supinely.30 Moreover, the incidence of clinically diagnosed VAP among patients positioned prone, does not differ significantly from the incidence of clinically diagnosed VAP among patients positioned supine.31,32
Kinetic Beds
Critical patients often for a long time remain immobile in the supine position so the functional residual capacity is decreased because of alveolar closure in dependent lung zones and impaired mucociliary clearance. This leads to the accumulation of mucus, atelectasis onset and ensuing infection.33 Rotational therapy uses a special bed designed to turn continuously, or nearly continuously, the patient from side to side; specific designs include kinetic therapy and continuous lateral rotation therapy (CLRT).34,35
Artificial Airway Management
Oral vs Nasal Intubation: Both nasogastric and nasotracheal tubes can cause oropharyngeal colonization and nosocomial sinusitis. Thus, use of the oral route for both endotracheal and gastric intubation should be considered to decrease the risk of VAP.36
Endotracheal tube cuff pressure: The secretions that pool above inflated endotracheal tube cuffs may be a source of aspirated material and ensuing VAP. The pressure of the endotracheal tube cuff should be optimized in order to prevent the leakage of colonized subglottic secretions into the lower airways. Persistent pressures into the tube cuff below 20 cm H2O have been associated with the development of VAP.37
Silver-Coated Endotracheal Tubes: Silver-coated endotracheal tubes appear to be safe, reduces bacterial biofilm formation, has bactericidal activity, reduces bacterial burden and can delay airway colonization. However, further studies are needed to for determing its efficacy.38,39
Mechanical Ventilation Management
Ventilator Circuit Change: The CDCs recommendation was ‘do not change routinely, on basis of duration of use, the breathing circuit that is in use on an individual patient. Change the circuit when it is visibly soiled or mechanically malfunctioning.40
Humidification With Heat and Moisture Exchangers: The effect of HME in preventing VAP is still controversial and recent studies have failed to show a significant difference in rates of infection.41
Subglottic secretion drainage: Intermittent subglottic secretions drainage using inspiratory pause during mechanical ventilation results in a significant reduction in VAP.42 SSD reduces VAP in patients ventilated for >72 hours and should be considered with other recommended strategies such as semi-recumbent positioning.43
Pharmacologic Strategies
Modulation of Oropharyngeal Colonization
Policies encouraging routine tropical oral decontamination with chlorhexidine for patients merit reevaluation. It is a cheap measure, but whether is it a safe one − it does not select resistant microorganisms − remains to be investigated.8,44
Selective Decontamination of the Digestive Tract
Selective decontamination of the digestive tract (SDD) is the decontamination ofpotentially pathogenic microorganisms living in the mouth and stomach, whilst preserving the indigenous anaerobic flora. SDD is an effective and safe preventive measure in ICUs where incidence rates of MRSA and VRE are low, but in ICUs with high rates of multi-resistant microorganisms it is a measure that is effective but not safe.45,46
Stress Ulcer Prophylaxis
Patients at risk from important gastrointestinal bleeding (shock, respiratory failure requiring mechanical ventilation or coagulopathy) should receive H2 antagonists such as ranitidine rather than sucralfate.47
Ventilator sedation protocol
In patients receiving mechanical ventilation and requiring sedative infusions with midazolam or propofol, the use of a nurse-implemented sedation protocol decreases the rate of VAP and the duration of mechanical ventilation.48 An objective assessment-based Analgesia-Delirium-Sedation (ADS) protocol without daily interruption of medication infusion decreases ventilator days and hospital length of stay in critically ill trauma patients.49
Antibiotic Policy and Infection Control
Rational antibiotic policy is a key issue for better patient care and preventing antimicrobial resistance.50,51 Infection control programs like using a scheduled switch of antibiotic class have demonstrated efficacy in reducing nosocomial infection rates and restraining multidrug resistant (MDR) microorganism emergence.52
VAP prevention in low resource/developing countries
Though the incidence of VAP has declined in the developed countries, it continues to be unacceptably high in the developing world. Its incidence in these countries is 20 times that in the developed nations with significant morbidity, mortality, and increase in ICU length of stay, which may represent an additional burden on the scarce resources in developing countries.53 Insufficient preventive strategies and probably inappropriate antibiotics administration may have lead to this scenario. Since microbiology and resistance pattern in India is different from other countries, there is need for data from our country to choose appropriate antimicrobials for management.54 Simple and effective preventive measures can be instituted easily and at minimal costs. Such measures might include hand hygiene, diligent respiratory care, elevation of head, oral and not nasal cannulation, minimization of sedation, institution of weaning protocols, judicious antibiotics use, de-escalation, and leveraging PK/PD characteristics for antibiotics administered. More costly interventions should be reserved for appropriate situations. Strategies to prevent VAP, probably by emphasis on practical, low-cost, low technology, easily implemented measures is need of the hour.
Ventilator-associated events (VAE) surveillance: an objective patient safety opportunity
Surveillance for ventilator-associated pneumonia is challenging and contains many subjective elements, including the use of chest x-ray evidence of pneumonia. In January 2013, CDC convened a VAP Surveillance Definition Working Group which transitioned VAP surveillance to ventilator-associated event (VAE) surveillance in adult inpatient settings.55 The VAE algorithm—which is a surveillance algorithm and not intended for use in the clinical management of patients—consists of 3 tiers of definitions: Tier 1, Ventilator-Associated Conditions (VAC); Tier 2, Infection -related Ventilator-Associated Complications (IVAC); and Tier 3, Possible and Probable VAP.27 The tier 1, VAC attempts to identify sustained respiratory deterioration episodes, and capture both infectious and noninfectious conditions and complications occurring in patients receiving mechanical ventilation. The tier 2, IVAC, is intended to identify the subset of VACs that are potentially related to pulmonary and extra pulmonary infections of sufficient severity to trigger respiratory deterioration. The tier 3, possible and probable VAP, attempts to identify IVAC patient subsets with respiratory infections as manifested by objective evidence of purulent respiratory secretions (where purulence is defined by using quantitative or semi-quantitative criteria for the number of neutrophils on Gram stain) and/or positive results of microbiological tests done on respiratory specimens. Because of the wide range of the lower respiratory tract specimens, their collection procedure as well as in laboratory processing and reporting of results, the Working Group of CDC determined that it was not appropriate to include these data elements in the VAC and IVAC definitions.56
This 3 tier approach is ineffective to accurately identify VAP for surveillance purposes and focuses on more mechanical ventilation complications. This approach may also reduce the likelihood of manipulation that could artificially lower event rates. Most VAEs are caused by pneumonia, pulmonary edema, atelectasis, or acute respiratory distress syndrome. In few recent studies concordance between the VAE algorithm and VAP was found to be poor.57 Thus, more studies are needed to further validate VAE surveillance compared with conventional VAP by using strong microbiologic criteria, particularly bronchoalveolar lavage with a protected specimen brush for diagnosing VAP and to better characterize the clinical entities underlying VAE.
Bundle approach to prevention of VAP
One of the five goals of the ‘Saving 100,000 Lives’ campaign, launched by the Institute for Healthcare Improvement is to prevent VAP and deaths associated with it by implementing a set of interventions for better patient care known as the ‘ventilator bundle’. The interventions should have scientific support of effectiveness, based on randomized controlled trials. All the elements of the bundles must be executed at the same time. The bundles for VAP includes four components: (a) elevation of the head end of the bed to 30-45º, (b) daily interruption of sedation, (c) daily assessment of readiness to extubate and (d) prophylaxis for deep venous thrombosis and peptic ulcer disease. The bundle approach to prevention of VAP has been found to be highly effective in reducing the incidence, mortality and ICU stay.5,58,59 The ventilator bundle should be modified and expanded to include specific processes of care that have been definitively demonstrated to be effective in VAP reduction. A multidimensional framework with a long-lasting program can successfully increase compliance with preventive measures directly dependent on healthcare workers bedside performance.
CONCLUSION
Ventilator Associated Pneumonia is one of the most common nosocomial infections in ICU presenting with non specific symptoms and clinical signs. Quantitative culture obtained by different methods, including EA, BAL, pBAL, PSB or TBA seem to be rather equivalent in diagnosing VAP. Clinical criteria used in combination, may be useful in VAP diagnosis; however, inter-observer variability and the moderate performance are to be considered.
Preventive strategies should focus on better secretion management and on reduction in bacterial colonization. Further research on targeted interventions is needed to effectively reduce VAP incidence. For VAP an approach based on multidisciplinary group is required including setting preventive benchmarks, establishing goals and time lines and providing appropriate education and training, audits and feedback to the staff, while continually updating themselves based on relevant clinical and preventive strategies.
|
Acknowledgements NIL Competing Interests None declared Author Details VARUN GOEL MD Microbiology, AIIMS, New Delhi, India; SAVITA GUPTA MD Anaesthesia, LNJP, New Delhi, India; TARUN GOEL MRCP, HOLY FAMILY HOSPITAL, New Delhi, India. CORRESPONDENCE: Dr Varun Goel, Senior Resident, Clinical Microbiology division, Department of Laboratory Medicine, AIIMS, New Delhi-110029. Email: drvarun21@gmail.com |
References
- Charles MP, Kali A, Easow JM, Joseph NM, Ravishankar M, Srinivasan S,et al. Ventilator-associated pneumonia. Australas Med J 2014; 7:334-44.
- Tejerina E, Frutos-Vivar F, Restrepo MI, Anzueto A, Abroug F, Palizas F, et al. Incidence, risk factors, and outcome of ventilator-associated pneumonia. J Crit Care 2006;21:56–65.
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Gupta A, Kapil A, Kabra SK, Lodha R, Sood S, Dhawan B, et al. Assessing the impact of an educational intervention on ventilator-associated pneumonia in a pediatric critical care unit. Am J Infect Control 2014; 42:111–5.
- Craven DE. Preventing Ventilator-Associated Pneumonia in Adults. Chest 2006; 130:251-60.
- Park DR. The Microbiology of Ventilator-Associated Pneumonia. Respir Care 2005;50: 742–65.
- Goel V, Hogade SA, Karadesai S. Ventilator associated pneumonia in a medical intensive care unit: Microbial aetiology, susceptibility patterns of isolated microorganisms and outcome. Indian J Anaesth 2012;56:558–62.
- Conway MA, Kefala K, Wilkinson TS, Dhaliwal K, Farrell L, Walsh T, et al. C5a mediates peripheral blood neutrophil dysfunction in critically ill patients. Am J Respir Crit Care Med 2009;180:19-28.
- Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R. Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171:955-63.
- Koenig SM, Truwit JD. Ventilator-Associated Pneumonia: Diagnosis, Treatment, and Prevention. Clin Microbiol Rev 2006;19:637–57.
- Fartoukh M, Maitre B, Honore S, Cerf C, Zahar JR, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003; 168:173-9.
- Shan J, Chen HL, Zhu JH. Diagnostic accuracy of clinical pulmonary infection score for ventilator-associated pneumonia: a meta-analysis. Respir Care 2011;56:1087-94.
- Pham TN, Neff MJ, Simmons JM, Gibran NS, Heimbach DM, Klein MB. The clinical pulmonary infection score poorly predicts pneumonia in patients with burns. J Burn Care Res Off Publ Am Burn Assoc 2007;28:76–9.
- Bhatia M, Zemans RL, Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2012;46:566-72.
- Rea-Neto A, Youssef NCM, Tuche F, Brunkhorst F, Ranieri VM, Reinhart K, et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care 2008;12:R56.
- Rello J. Bench-to-bedside review: Therapeutic options and issues in the management of ventilator-associated bacterial pneumonia. Crit Care Lond Engl 2005;9:259–65.
- Sierra R, Benítez E, León C, Rello J. Prevention and diagnosis of ventilator-associated pneumonia: a survey on current practices in Southern Spanish ICUs. Chest 2005;128: 1667–73.
- Magret M, Lisboa T, Martin-Loeches I, Manez R, Nauwynck M, Wrigge H, et al. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: a prospective and observational multicenter study. Crit Care. 2011;15:R62.
- Khilnani G, Arafath TKl, Hadda V, Kapil A, Sood S, Sharma S. Comparison of bronchoscopic and non-bronchoscopic techniques for diagnosis of ventilator associated pneumonia. Indian J Crit Care Med 2011;15:16.
- Scholte JBJ, van Dessel HA, Linssen CFM, Bergmans DCJJ, Savelkoul PHM, Roekaerts PMHJ, et al. Endotracheal aspirate and bronchoalveolar lavage fluid analysis: interchangeable diagnostic modalities in suspected ventilator-associated pneumonia? J Clin Microbiol. 2014;52:3597–604.
- Nair S, Sen N, Peter JV, Raj JP, Brahmadathan KN. Role of quantitative endotracheal aspirate and cultures as a surveillance and diagnostic tool for ventilator associated pneumonia: a pilot study. Indian J Med Sci 2008;62:304–13.
- Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care 2005;50:725–39; discussion 739–41.
- Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care 2014;18:208.
- Chastre J, Luyt CE, Combes A, Trouillet JL. Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator-associated pneumonia to decrease resistance in the intensive care unit. Clin Infect Dis 2006 ;43 Suppl 2:S75-81.
- Lalwani S, Mathur P, Tak V, Janani S, Kumar SI, Bagla R, et al. Diagnosis of ventilator-associated pneumonia: comparison between ante-mortem and post-mortem cultures in trauma patients. Indian J Med Microbiol 2014; 32:294–300.
- Albert M, Friedrich JO, Adhikari NKJ, Day AG, Verdant C, Heyland DK, et al. Utility of Gram stain in the clinical management of suspected ventilator-associated pneumonia. Secondary analysis of a multicenter randomized trial. J Crit Care 2008;23:74–81.
- Magill SS, Klompas M, Balk R, Burns SM, Deutschman CS, Diekema D, et al. Developing a new, national approach to surveillance for ventilator-associated events. Crit Care Med 2013;41:2467–75.
- Crnich CJ, Safdar N, Maki DG. The role of the intensive care unit environment in the pathogenesis and prevention of ventilator-associated pneumonia. Respir Care 2005;50: 813–36.
- Mathur P. Hand hygiene: Back to the basics of infection control. Indian J Med Res 2011 ;134: 611–20.
- Alexiou VG, Ierodiakonou V, Dimopoulos G, Falagas ME. Impact of patient position on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. J Crit Care 2009;24:515–22.
- Li Bassi G, Torres A. Ventilator-associated pneumonia: role of positioning. Curr Opin Crit Care 2011;17:57-63
- Tinti M, Gracias V, Kaplan LJ. Adjuncts to ventilation part II: monitoring, fluid management, bundles, and positioning. Curr Probl Surg 2013;50:433-7.
- Delaney A, Gray H, Laupland KB, Zuege DJ. Kinetic bed therapy to prevent nosocomial pneumonia in mechanical ventilated patients: A systematic review and meta analysis. Crit Care 2006; 10: R70.
- Hess DR. Patient Positioning and Ventilator-Associated Pneumonia. Respir Care 2005; 50: 892–9.
- Culpepper SL, Skaggs RL, Vangilder CA. The impact of continuous lateral rotation therapy in overall clinical and financial outcomes of critically ill patients. Crit Care Nurs Q 2008; 31:270-9.
- Blot SI, Poelaert J, Kollef M. How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infect Dis 2014; 14:119.
- Hamilton VA, Grap MJ. The role of the endotracheal tube cuff in microaspiration. Heart Lung J Crit Care 2012; 41:167–172 .
- Rello J, Kollef M, Diaz E, Sandiumenge A, Castillo Y del, Corbella X, et al. Reduced burden of bacterial airway colonization with a novel silver-coated endotracheal tube in a randomized multiple-center feasibility study. Crit Care Med 2006; 34:2766–72.
- Haas CF, Eakin RM, Konkle MA, Blank R. Endotracheal tubes: old and new. Respir Care 2014; 59:933-52; discussion 952-5.
- Han J, Liu Y. Effect of ventilator circuit changes on ventilator-associated pneumonia: a systematic review and meta-analysis. Respir Care 2010; 55:467-74.
- Kelly M, Gillies D, Todd DA, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Anesth Analg 2010;111:1072.
- Safdari R, Yazdannik A, Abbasi S. Effect of intermittent subglottic secretion drainage on ventilator-associated pneumonia: A clinical trial. Iran J Nurs Midwifery Res 2014; 19: 376–80.
- Leasure AR, Stirlen J, Lu SH. Prevention of ventilator-associated pneumonia through aspiration of subglottic secretions: a systematic review and meta-analysis. Dimens Crit Care Nurs 2012; 31:102-17.
- Sebastian MR, Lodha R, Kapil A, Kabra SK. Oral mucosal decontamination with chlorhexidine for the prevention of ventilator-associated pneumonia in children - a randomized, controlled trial. Pediatr Crit Care Med 2012; 13:e305-10.
- Bonten MJM. Selective digestive tract decontamination--will it prevent infection with multidrug-resistant gram-negative pathogens but still be applicable in institutions where methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci are endemic? Clin Infect Dis Off Publ Infect Dis Soc Am 2006;43:S70–4.
- Keyt H, Faverio P, Restrepo MI. Prevention of ventilator-associated pneumonia in the intensive care unit: a review of the clinically relevant recent advancements. Indian J Med Res 2014; 139:814-21.
- Isakow W, Kollef MH. Preventing ventilator-associated pneumonia: an evidence-based approach of modifiable risk factors. Semin Respir Crit Care Med 2006;27:5-17.
- Quenot JP, Ladoire S, Devoucoux F, Doise JM, Cailliod R, Cunin N,et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med 2007;35:2031-6.
- Robinson BR, Mueller EW, Henson K, Branson RD, Barsoum S, Tsuei BJ. An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. J Trauma 2008;65:517-26.
- Mathur P, Singh S. Multidrug Resistance in Bacteria: A Serious Patient Safety Challenge for India. J Lab Physicians 2013;5:5-10.
- Chawla R.Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries.Am J Infect Control 2008 ;36:S93-100.
- Raineri E, Crema L, Dal Zoppo S, Acquarolo A, Pan A, Carnevale G, et al. Rotation of antimicrobial therapy in the intensive care unit: impact on incidence of ventilator-associated pneumonia caused by antibiotic-resistant Gram-negative bacteria. Eur J Clin Microbiol Infect Dis 2010;29:1015-24.
- Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Anttila A, et al. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am J Infect Control 2013; 41:286-300.
- Khilnani G C, Jain N. Ventilator-Associated pneumonia: Changing microbiology and implications. Indian J Crit Care Med 2013; 17:331-2.
- Raoof S, Baumann MH. Ventilator-Associated Events: The New Definition. Am J Crit Care January 2014;23:7-9.
- Klein Klouwenberg PM, van Mourik MS, Ong DS, Horn J, Schultz MJ, Cremer OL, et al. Electronic implementation of novel surveillance paradigm for ventilator-associated events. Feasibility and validation. Am J Respir Crit Care Med. 2014;189:947-55.
- Lilly CM, Landry KE, Sood RN, Dunnington CH, Ellison RT, Bagley PH et al. Prevalence and test characteristics of national health safety network ventilator-associated events. Crit Care Med. 2014; 42:2019-28.
- Eom JS, Lee MS, Chun HK, Choi HJ, Jung SY, Kim YS, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: a multicenter study. Am J Infect Control. 2014;42:34-7.
- Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010; 25:174.e11-8.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




