Case Report: Group B Streptoccocus Related Lower Limb Necrotizing Fasciitis, Complicated by Purulent Pericarditis and Cardiac Tamponade
Raja Ezman Faridz Raja Shariff, Rizmy Najme Khir & Sazzli Kasim
Cite this article as: BJMP 2019;12(1):a001
|
|
Abstract Background: Necrotizing soft tissue infections (NSTI) are severe and rapidly progressive. Rarely, Group B Streptococcus (GBS) can cause NSTI, majority due to an immunocompromised state. Even more uncommon is pericardial involvement following NSTI of a non-adjacent structure. Abbreviations: GBS: Group B Streptoccocus ECG: Electrocardiogram |
Background
Necrotising soft tissue infections (NSTI) are severe and rapidly progressive, requiring rapid recognitions and early, often surgically-based, management. Mono-microbial types of NSTI (i.e. Type 2 NSTI), which amounts for 20 to 30% of overall cases, are often linked to invasive Group A Streptococcus or Staphylococcus Aureus infections 1. Rarely, Group B Streptoccocus (GBS), also known as Streptococcus Agalactiae, are implicated 2. We report a unique case of NSTI of the lower limbs due to GBS, with acute pericardial dissemination leading to cardiac tamponade, leading to a diagnostic dilemma due to co-existing cardiogenic and septic shock.
Case Report
A 51-year old gentleman of Chinese ethnicity presented with right foot pain and swelling over 2 weeks, associated with chest pain and shortness of breath during that period. He had a 10-year history of poorly controlled diabetes mellitus with a Hba1c level of 8.8 %, hypertension and dyslipidaemia.
He was hypotensive on arrival, with a blood pressure of 91/60 mmHg and hypoxic, requiring high flow oxygen of 15L/min to maintain saturations at 100%. Otherwise other vitals were stable, pulse rate being 72 beats per minutes, respiratory rate 24 breaths per minute and a temperature of 37.4 degrees Celsius.
Clinical examination revealed a gangrenous lateral two toes extending into the lateral malleolus on the right foot, with evidence of pus discharge and associated warmth and crepitus up to hindfoot level on palpation. There was also evidence of dry gangrene in the fourth toe of the left foot, with presence of a small puncture at dorsum of foot with pus discharge. Similarly, crepitus was felt up to midfoot level on palpation of the left side. Bilateral dorsalis pedis and posterior tibialis pulses were palpable but feeble.
Table 1: Blood Investigations on Admission
| Blood Test | Results | Blood Test | Results |
| White Cell Count | 26.99 x109L | Alkaline Phosphatase | 168 U/L |
| Neutrophil | 90.30% | Alanine Aminotransferase | 37 U/L |
| Lymphocyte | 4.50% | Aspartate Aminotransferase | 40 U/L |
| Platelets | 210 x109L | Sodium | 121 mmol/L |
| Hemoglobin | 10.0 g/dL | Pottasium | 7.6 mmol/L |
| Lactate Dehydrogenase | 441 U/L | Urea | 40.5 mmol/L |
| International Normalised Ratio | 1.2 | Creatinine | 323 μmol/L |
| Activated Partial Thromboplastin Time | 36.5 s | Creatinine Kinase | 43 U/L |
| Prothrombin Time | 14.6 s | Total Bilirubin | 21 μmol/L |
Figure 1a & 1b: Radiography of the (1a) left foot and (1b) right foot respectively, demonstrating gas within soft tissue bilaterally.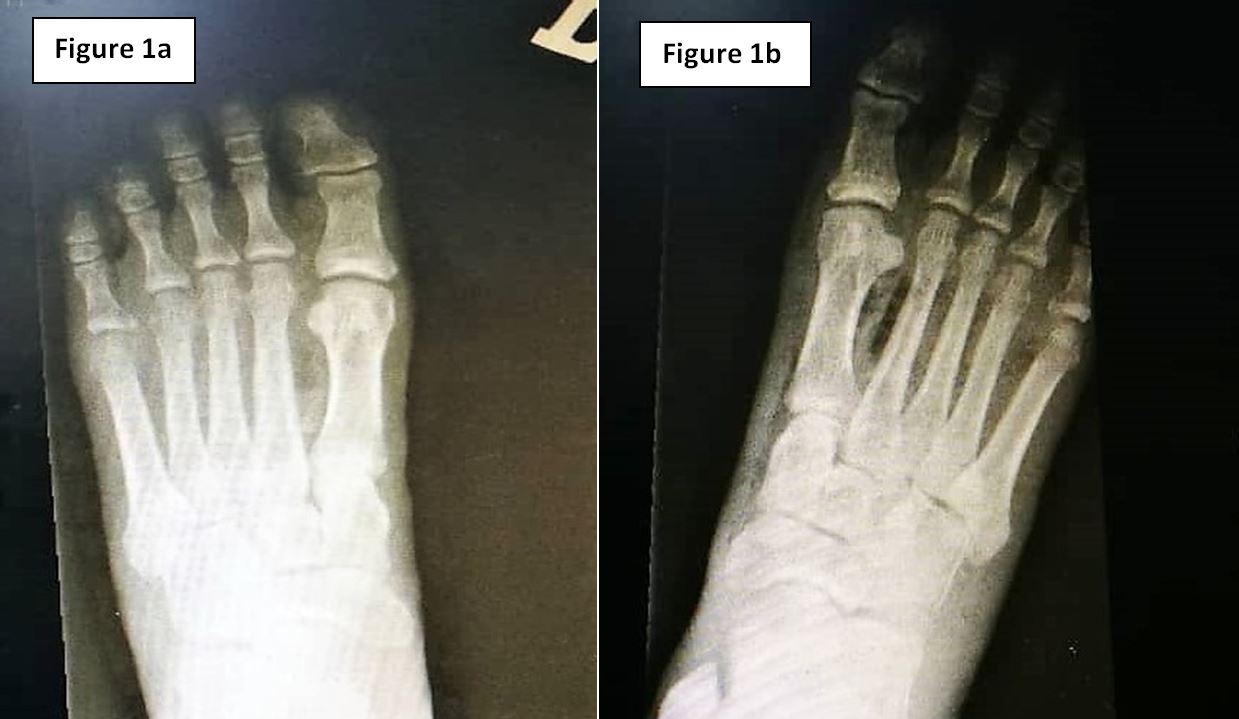
Figure 2: Chest radiography demonstrating cardiomegaly and globular-shaped heart, with loss of left-sided cardiophrenic and costophrenic angles.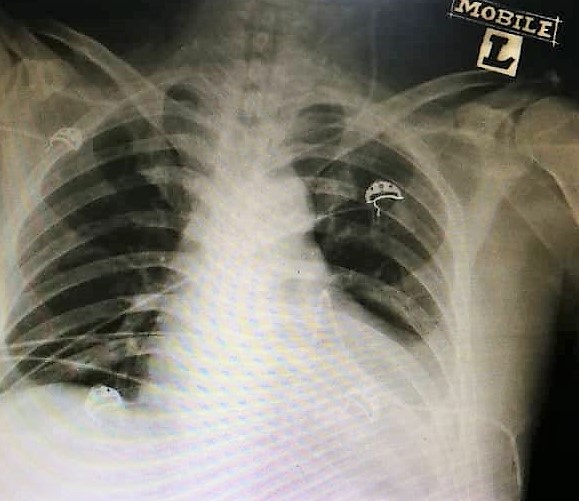
Figure 3: Electrocardiogram demonstrating widespread saddle-shaped ST-elevation, consistent with pericarditis.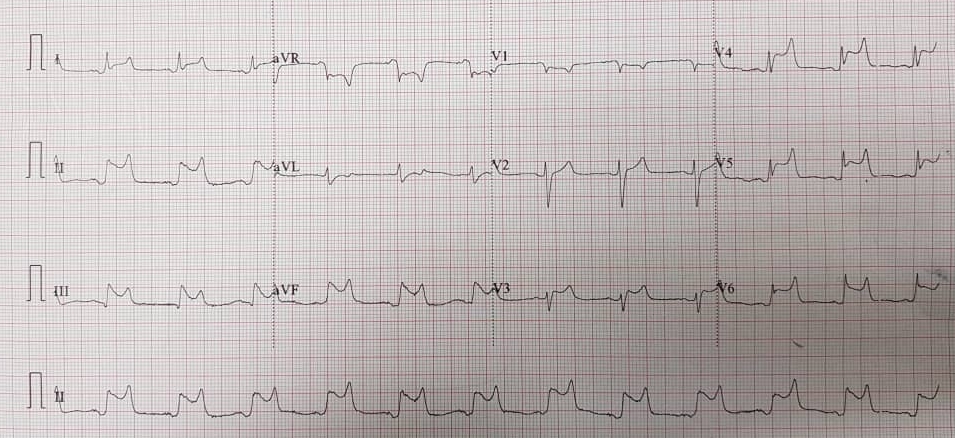
Figure 4: Parasternal Long Axis view of bedside echocardiography showing evidence of pericardial effusion and right atrial and ventricular collapse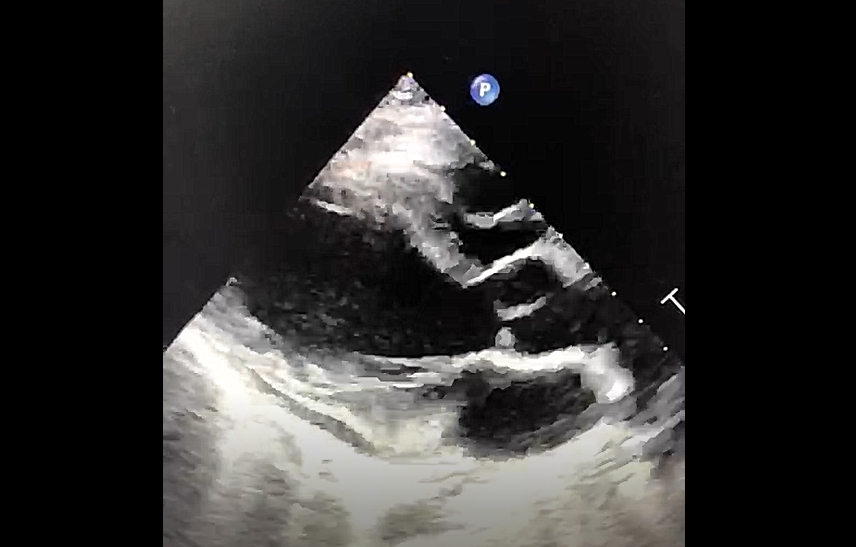
Figure 5: Purulent pericardial aspirate via pericardiocentesis
Initial blood investigations are highlighted in Table 1. HIV Antibody, Hepatitis B Surface Antigen and Hepatitis C Antibody serology were all negative. Lower limb radiography revealed evidence of gaseous shadows bilaterally (Figure 1). The clinical and radiological findings were consistent with necrotising soft tissue infection of bilateral feet, and the patient was advised for extensive wound debridement and possible amputation of the affected sites during an orthopaedic consult.
However, on closer review of the chest radiography, there was evidence of cardiomegaly with a globular-shaped heart (Figure 2). His electrocardiogram on arrival, revealed diffuse ST-segment elevations on majority of leads, ST-segment depression on lead aVR consistent with pericarditis (Figure 3).
A bedside echocardiogram was performed, revealing a massive pericardial effusion, measuring largest at 2 cm in depth, with evidence of both right atrial and ventricular collapse (Figure 4).
An urgent pericardiocentesis was performed, under echocardiographic guidance, which revealed turbid-looking aspirate (Figure 5). Urgent microscopic analysis revealed 45 Cells per mm3, majority of which were lymphocytes, and gram stain showed moderate amounts of pus cells with occasional gram positive cocci. Pericardial fluid was negative for acid-fast bacilli.
A repeat transthoracic echocardiogram was performed post-pericardial drain insertion, revealing minimal remnant pericardial fluid, with the pericardial drain in-situ, and no evidence of any mass or vegetation. Unfortunately, a transoesophageal echocardiography and Computed Tomography (CT) imaging of the mediastinum (to rule out mediastinitis and pneumonitis) was not performed, as management of the NSTI took precedence.
The patient was started on intravenous antibiotics, both tazobactom-pipericillin and clindamycin. There was a delay in performing limb saving wound debridement as the patient was reluctant for invasive management, but had later consented to the procedure which was performed only on day 3 of admission. Tissue cultures were taken peri-operatively. Unfortunately, the patient deteriorated post-operatively due to extensive blood loss and overwhelming septicaemia and succumbed to his illness 72 hours after. Subsequently, it was revealed that cultures from blood, pericardial aspirate and tissue aspirate were positive for GBS infection.
Discussion
GBS is a common microorganism, often colonising the gastrointestinal and reproductive tract 3. Rarely, GBS colonises the skin and can cause necrotising fasciitis, i.e. necrotising soft tissue infection (NSTI), with only 22 cases having been reported in the past ii. Majority of these patients are either immunocompromised or have other predisposing factors including recent thoracic intervention or trauma 4, 5.
GBS-related infections of cardiac structures are rare, as a whole, with 2 to 3% of cases presenting as native valve endocarditis and far less as pericarditis, mycotic aneurysms and intraventricular abscesses 3. Parikh et al reviewed the types of microorganisms isolated from purulent pericarditis samples and revealed that only 5% were due to streptococcal organism, sans Streptococcus Pneumoniae, possibly less so due to GBS 6. Our literature search revealed only one case of GBS-related purulent pericarditis reported although the case was not linked in any way to a NSTI to our knowledge 7.
Our case was unique as, at the time of writing, there were no other reports of GBS-related lower limb NSTI in combination with mediastinal involvement. There have been only a handful of cases of necrotising fasciitis reported with mediastinal involvement, the majority of which were supra-diaphragmatic with only one reporting NSTI of the lower limb due to Aspergillus infection 8 9.
The similarity in culture results obtained from blood, tissue aspirate and the pericardial fluid in our patient suggest dissemination of GBS from the NSTI, possibly via a haematogenous route, although bacterial-related pericardial dissemination can also occur via direct spread from infected foci from neighbouring intra-thoracic structures or sub-diaphragmatic 4. The possibility of multi-routed spread should remind clinicians that, albeit rare, mediastinal involvement in NSTI is a possible complication of such disease.
Conclusion
This case highlights the rare possibility of cardiac involvement in cases of NSTI, and the possibility of cardiac tamponade causing cardiogenic shock masquerading alongside septic shock. It also highlights the importance of combining clinical findings with ancillary testing, including bedside echocardiography, when faced with challenging cases of sepsis to help look for possible foci of infection.
|
Competing Interests None declared Author Details RAJA EZMAN FARIDZ RAJA SHARIFF (MBCHB, MRCP), Universiti Teknologi Mara, Sungai Buloh, Malaysia. RIZMY NAJME KHIR (MBBCH, MRCP), Universiti Teknologi Mara, Sungai Buloh, Malaysia. SAZZLI KASIM (MBBCh, BAO, BMedSc, MRCPI, CSCST, FNHAM), Universiti Teknologi Mara, Sungai Buloh, Malaysia. CORRESPONDENCE: RAJA EZMAN FARIDZ RAJA SHARIFF, Universiti Teknologi Mara Sungai Buloh Campus, Jalan Hospital, Sungai Buloh, 47000, Selangor, Malaysia. Email: rajaezman@gmail.com |
References
- Bonne SL & Kadri SS. Evaluation and Management of Necrotizing Soft Tissue Infections. Infect Dis Clin N Am. 2017. 31: 497–511
- Fukuda K, Ryujina M, Sakiod R, Satoshi S, Takanori F, et al. Bilateral Necrotizing Fasciitis of the Foot Associated with Group B Streptococcus. Case Rep Dermatol. 2016. 8:243–249
- Hirai N, Kasahara K, Uno K, Ogawa Y & Ogawa T. Infective Endocarditis Complicated by Intraventricular Abscesses, Pericarditis, and Mycotic Aneurysm Due to an Emerging Strain of Serotype VI Streptococcus agalactiae. Jpn. J. Infect. Dis. 2017. 70: 685–686
- Imaziao M, Brcatob A, DeRosac FG, Lestuzzid C, Bombanab E, et al. Aetiological diagnosis in acute and recurrent pericarditis: when and how. J Cardiovasc Med. 2009. 10: 217–230
- Wong CH, Kurup A & Tan KC. Group B Streptococcus necrotizing fasciitis: an emerging disease? Eur J Clin Microbiol Infect Dis. 2004. 23 (7): 573-5
- Parikh SV, Memon N, Echols M, Shah J, McGuire DK & Keely EC. Purulent Pericarditis Report of 2 Cases and Review of the Literature. Medicine. 2009. 88: 52-65
- Karim MA, Bach RG, Dressler F, Caracciolo E, Donohue T & Kern MJ. Purulent pericarditis caused by group B streptococcus with pericardial tamponade. AMHEARTJ. 1993. 126: 727-730
- Sergi C, Weitz J, Hofmann WJ, Sinn P, Eckart A, et al. Aspergillus endocarditis, myocarditis and pericarditis complicating necrotizing fasciitis. Case report and subject review. Virchows Arch. 1996. 429: 177-180
- Lalwani AK & Kaplan MJ. Mediastinal and Thoracic Complications of Necrotzing Fasciitis of the Head and Neck. Head & Neck. 1991. 13: 531-539

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




