Helen Laycock and Abid Rajah
Abstract
Acute lung injury is a syndrome with a diagnostic criteria base on hypoxaemia and a classical radiological appearance, with acute respiratory distress syndrome at the severe end of the disease spectrum. Its incidence is common, it is likely to exist outside the intensive care setting and therefore is a condition relevant to all clinicians. Genetically predisposed individuals are subject to environmental triggers which can be intra or extrapulmonary in nature. An inflammatory response causes damage to alveolar epithelial cells and vasculature, impairing gas exchange and can lead to multiple organ failure. Management centres around supportive care and treating the cause, but evidence supports use of low tidal volume ventilatory settings and conservative intravenous fluid strategies. Long term outcomes are related to neuromuscular, cognitive and psychological issues rather than pulmonary, and rehabilitation during recovery needs to focus on this.
|
Acute Lung Injury (ALI) is a continuum of clinical and radiographic changes affecting the lungs, characterised by acute onset severe hypoxaemia, not related to left atrial hypertension, occurring at any age. At the severe end of this spectrum lies Acute Respiratory Distress Syndrome (ARDS) and therefore unless specifically mentioned this review will address ARDS within the syndrome of ALI.
It was first described by Ashbaugh in the Lancet in 1967. This landmark paper described a group of 12 patients with “Respiratory Distress Syndrome” who had refractory hypoxaemia, decreased lung compliance, diffuse infiltrates on chest radiography and required positive end expiratory pressure (PEEP) for ventilation.1
|
Key Points on Acute Lung Injury
- Common, life threatening condition which is a continuum of respiratory dysfunction with ALI and ARDS being at either end of the spectrum
- Risk factors include conditions causing direct and indirect lung injury, leading to an inflammatory response which can cause multiple organ failure
- Damage to alveolar epithelial cells and capillary vasculature impair gas exchange and can lead to fibrosis
- Management aims include supportive care, maintaining oxygenation and diagnosing and treating the underlying cause
- Evidence supports low tidal volume ventilation and conservative fluid management
- Long term outcomes relate to neuromuscular, neurocognitive and psychological problems rather than pulmonary dysfunction
|
This initial description gave only vague criteria for diagnosis, focused on the most severe end of the continuum and was not specific enough to exclude other conditions. A more precise definition was described by Murray et al. in 1988 using a 4 point lung injury scoring system including the level of PEEP used in ventilation, ratio of arterial oxygen tension to fraction of inspired oxygen (PaO₂/FiO₂), static lung compliance and chest radiography changes
2. Despite being more specific and assessing severity it was too large and complex for practical purposes in the ICU setting.
It was not until 1994 that The American –European Consensus Conference on ARDS set the criteria used today to define both ALI and ARDS in research and clinical medicine. It recommended ALI be defined as “a syndrome of inflammation and increased permeability that is associated with a constellation of clinical, radiological and physiological abnormalities that cannot be explained by, but may coexist with, left atrial or pulmonary capillary hypertension” .
3 They distinguished between ALI and ARDS based upon the degree of hypoxaemia present, as determined by the ratio of partial pressure of arterial oxygen to fractional inspired oxygen concentration (PaO₂/FiO₂), with ALI patients demonstrating a milder level of hypoxaemia. Additionally ARDS changed from Adult Respiratory Distress Syndrome to Acute Respiratory Distress Syndrome to account for its occurrence at all ages.
DIAGNOSIS AND PROBLEMS RELATED TO THIS
There are no gold standard radiological, laboratory or pathological tests to diagnosis ALI and ARDS and patients are given the diagnosis based on meeting the criteria agreed in 1994. (See Table 1)
ALI is diagnosed clinically and radiologically by the presence of non-cardiogenic pulmonary oedema and respiratory failure in the critically ill.
Table 1 – Diagnostic Criteria for ALI and ARDS
|
|
ALI
|
ARDS
|
|
Onset
|
Acute
|
Acute
|
|
Oxygenation (PaO₂/FiO₂) ratio in mmHg, regardless of ventilatory settings
|
<300
|
<200
|
|
Chest Radiological Appearance
|
Bilateral Pulmonary Infiltrations which may or may not be symmetrical
|
Bilateral Pulmonary Infiltrations which may or may not be symmetrical
|
|
Pulmonary Wedge Pressure
(in mmHg)
|
<18 or no clinical evidence of left atrial hypertension
|
<18 or no clinical evidence of left atrial hypertension
|
Meeting criteria, in itself, is not a problem when diagnosing conditions in the ICU setting, as sepsis and multi-organ failure are defined using consensus based syndrome definitions, however there are problems specifically related to ALI’s diagnosis.
In practice ALI and ARDS are clinically under-diagnosed, with reported rates ranging between 20 to 48% of actual cases.
4 This is due to poor reliability of the criteria related to;
- Non-specific radiological findings which are subject to inter-observer variability
- Oxygenation criteria is independent of inspired oxygen concentration or ventilator settings including lung volumes and PEEP
- Excluding cardiac causes of pulmonary oedema including left ventricular failure, mitral regurgitation and cardiogenic shock, in the ICU setting is difficult even when pulmonary artery catheters are used
- The definition includes a heterogeneous population who behave very differently in response to treatment, duration of mechanical ventilation and severity of pulmonary dysfunction.
However this is the definition used by the ARDS network (a clinical network set up in 1994 by The National Heart, Lung and Blood Institute and the National Institutes of Health in the USA) for its clinical trials and on this basis it is validated.
EPIDEMIOLOGY
Incidence
Incidence of ALI is reported as 17-34 per 100,000 person years.
5 Unfortunately despite population studies demonstrating fairly consistent trends regarding age (mean approximately 60years), mortality (35-40%) and ratio of ARDS to ALI (around 70%), incidence figures are less consistent internationally. A recent prospective population-based cohort study in a single US county demonstrated a higher incidence around 78.9 per 100,000 person years and inferred from this that 190,600 cases could occur in the USA alone each year.
6 This variation is likely due to problems with reliability of diagnosis as illustrated above and also related to ALI generally presenting as a critical care illness making its epidemiology directly linked to availability of ICU resources.
Cases are only “captured” in the ICU setting and it potentially exists outside this environment in unknown quantities.
7 Taking this into account means ALI and ARDS are probably far commoner in clinical practice than reported and many patients may meet the diagnosis yet be managed outside the ICU environment.
8
Risk Factors
ALI is a multi-factorial process which occurs due to environmental triggers occurring in genetically predisposed individuals, as ALI-inducing events are common, yet only a fraction of those exposed develop the syndrome.
Environmental triggers for developing ALI can be divided into those causing direct and those causing indirect lung injury, with sepsis, either intrapulmonary or extrapulmonary being the commonest cause. (See table 2)
Table 2 Direct and Indirect triggers for ALI
|
Direct Lung Injury
|
Indirect Lung Injury
|
|
Common
Pneumonia
Aspiration of gastric contents
Less Common
Pulmonary contusion
Fat / Amniotic fluid embolism
High Altitude
Near Drowning
Inhalation Injury
Reperfusion Injury
|
Common
Sepsis
Severe trauma with shock and multiple transfusions
Less Common
Burns
Disseminated intravascular coagulation
Cardiopulmonary bypass
Drug overdose (heroin, barbiturates)
Acute pancreatitis
Transfusion of blood products
Hypoproteinaemia
|
At present there is research into the role of genetic factors and how they contribute to susceptibility and prognosis.
9 It is difficult to assess the molecular basis of ALI due to the range of ALI inducing events which can cause the lung injury, the heterogeneous nature of the syndrome itself, presence of additional comorbidities, potentially incomplete gene penetrance and complex gene-environment interactions. However possible candidate genes which predispose patients to ALI have been identified and other genes exist which may influence its severity, thus providing targets for research in treatment development.
Secondary factors including chronic alcohol abuse, chronic lung disease and low serum pH may increase risk of developing ALI.⁷ There may be factors which are protective against its development, such as diabetes in septic shock patients,
10 but further research is required.
PATHOPHYSIOLOGY
It is thought ALI patients follow a similar pathophysiological process independent of the aetiology. This occurs in two phases; acute and resolution, with a possible third fibrotic phase occurring in a proportion of patients.
Acute Phase
This is characterised by alveolar flooding with protein rich fluid secondary to a loss of integrity of the normal alveolar capillary base, with a heterogeneous pattern of alveolar involvement.
There are two types of alveolar epithelial cells (Table 3), both of which are damaged in ALI, likely via neutrophil mediation, with macrophages secreting pro-inflammatory cytokines, oxidants, proteases, leucotrienes and platelet activating factor.
Table 3 Characteristics of Type I and Type II Alveolar Epithelial Cells
|
|
Type I
|
Type II
|
|
Percentage of cells
|
90%
|
10%
|
|
Shape
|
Flat
|
Cuboidal
|
|
Function
|
Provide lining for alveoli
|
Replace damaged type I cells by differentiation
Produce surfactant
Transport ions and fluids
|
Damage to type I alveolar epithelial cells causes disruption to alveolar-capillary barrier integrity and allows lung interstitial fluid, proteins, neutrophils, red blood cells and fibroblasts to leak into the alveoli.
Damage to type II cells decreases surfactant production and that produced is of low quality, likely to be inactivated by fluid now in alveoli, which leads to atelectasis. Additionally there is impaired replacement of type I alveolar epithelial cells and an inability to transport ions and therefore remove fluid from the alveoli.
Coagulation abnormalities occur including abnormal fibrinolysis and formation of platelet and fibrin rich thrombi which result in microvascular occlusion, causing intrapulmonary shunting leading to hypoxaemia.
Ventilation-perfusion mismatch, secondary to alveolar collapse and flooding, decreases the number of individual alveoli ventilated, which in turn increases alveolar dead space, leading to hypercapnia and respiratory acidosis. Additionally pulmonary compliance decreases and patients start to hyperventilate in an attempt to compensate the above changes.
The release of inflammatory mediators from damaged lung tissue triggers systemic inflammation and systemic inflammatory response syndrome (SIRS) which may progress to multiple organ failure, a leading cause of death in ARDS patients.
Resolution Phase
This phase is dependent on repair of alveolar epithelium and clearance of pulmonary oedema and removal of proteins from alveolar space.
The type II alveolar epithelial cells proliferate across the alveolar basement membrane and then differentiate into type I cells. Fluid is removed by initial movement of sodium ions out of the alveoli via active transport in type II alveolar epithelial cells, with water then following, down a concentration gradient through channels in the type I alveolar epithelial cells.
Soluble proteins are removed by diffusion and non soluble proteins by endocytosis and transcytosis of type I alveolar epithelial cells and phagocytosis by macrophages.
Fibrotic Phase
Some patients do not undergo the resolution phase but progress to fibrosing alveolitis, with fibrosis being present at autopsy in 55% non-survivors of ARDS.
11 This occurs by the alveolar spaces filling with inflammatory cells, blood vessels and abnormal and excessive deposition of extracellular matrix proteins especially collagen fibres.
12 Interstitial and alveolar fibrosis develops, with an associated decrease in pulmonary compliance and only partial resolution of pulmonary oedema with continued hypoxaemia.
CLINICAL FEATURES
Acute Phase
The diagnosis should be considered in all patients with risk factors who present with respiratory failure, as the onset though usually over 12 to 72 hours, can be as rapid as 6 hours in presence of sepsis.
Patients present with acute respiratory failure where hypoxaemia is resistant to oxygen therapy and chest auscultation reveals diffuse, fine crepitations, indistinguishable from pulmonary oedema.
Resolution Phase
This phase usually occurs after around 7 days after onset of ALI, where a resolution of hypoxaemia and improvement in lung compliance is seen.
Fibrotic Phase
There is persistent impairment of gas exchange and decreased compliance. In severe cases it can progress to pulmonary hypertension through damage to pulmonary capillaries and even severe right heart failure, with the signs and symptoms of this developing over time.
INVESTIGATIONS
Diagnostic criteria require arterial blood gas analysis to demonstrate the required ratio between the partial pressure of arterial oxygen and fractional inspired oxygen concentration.
Radiological Findings
Although there are no pathognomonic radiographic findings for ALI, features on plain chest radiography include;
- Bilateral patchy consolidation, which may or may not be symmetrical
- Normal vascular pedical width
- Air bronchograms
- Pleural effusion may be present
- 10-15% patients have pneumothoraces independent of ventilator settings
Computer tomography of the chest can show the heterogeneous nature of ALI, with dependent areas of the lung showing patchy consolidation with air bronchograms, atelectasis and fibrosis. As with plain radiography there may be pneumothoraces present.

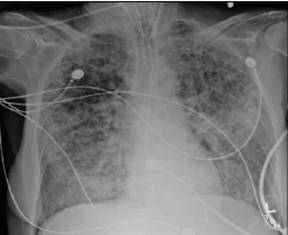
Computer tomography and Chest radiograph of ARDS
MANAGEMENT
The aims of management are to provide good supportive care, maintain oxygenation and to diagnose and treat the underlying cause.
General
Good supportive care, as for all ICU patients, should include nutritional support with an aim for early enteral feeding, good glycaemic control and deep venous thrombosis and stress ulceration prophylaxis. It is important to identify and treat any underlying infections with antibiotics targeted at culture sensitivities and if unavailable, towards common organisms specific to infection site.
It is not uncommon for ALI patients to die from uncontrolled infection rather than primary respiratory failure.
Ventilator associated pneumonia is common in patients with ALI and can be difficult to diagnoses, as ALI radiological findings can mask new consolidation and raised white cell count and pyrexia may already be present. If suspected this should be treated with appropriate antibiotics, although long term ventilation can cause colonisation which leads to endotracheal aspirate culture results being difficult to interpret.
Although the role of physiotherapy in ALI is unclear, aims of treatment should be similar to those in all ICU patients, including removal of retained secretions and encouragement of active and passive movements, as patients are often bed bound for prolonged periods of time.
Ventilation
MODE OF VENTILATION
Ventilation is usually via endotracheal intubation using intermittent positive pressure ventilation with PEEP. There may be a role for non invasive ventilation in early stages of ALI, but it is poorly tolerated at higher PEEP settings which may be required to maintain oxygenation, and no evidence supports its use at present. Additionally there is no evidence to suggest an advantage of either volume or pressure controlled ventilation.
Principles of ventilation in ALI are to maintain adequate gas exchange until cell damage resolves whilst avoiding ventilator associated injury from;
- Barotrauma – alveolar overdistension associated with ventilation at high volumes
- Volutrauma – alveolar overdistension associated with ventilator high pressures
- Biotrauma – repeated opening and closing of collapsed alveoli causing shearing stress which can initiate a proinflammatory process
Lungs in patients with ALI are heterogeneous and therefore can react variably to changes in ventilator settings. Therefore settings which provide adequate oxygenation, may damage more “healthy” areas of lung.
13
Table 4 Lung ventilation in different parts of lung with acute lung injury
|
Area
|
Characteristics
|
Behaviour when ventilated
|
|
1
|
Normal compliance and gas exchange
|
Easily over ventilated
Exposed to potential damage
|
|
2
|
Alveolar flooding and atelectasis
|
Alveoli can still be recruited for gas exchange by safely raising airway pressures
|
|
3
|
Severe alveolar flooding and inflammation
|
Alveoli cannot be recruited without using unsafe airway pressures
|
TIDAL VOLUMES
Old strategies of high volume ventilation are likely to over inflate healthy lung portions leading to barotrauma and ventilator management in ALI has moved towards lower tidal volumes. This is a consequence of the ARDSnet tidal volume study, which demonstrated significant reduction in mortality (40 to 31%) when using a low volume ventilator strategy based on predicted body weight (6mls/kg and peak pressures <30cmH₂O vs. 12mls/kg and peak pressures <50cmH₂O).
14 Furthermore they showed a decrease in systemic inflammatory markers, lower incidence of multiple organ failure and an increase in ventilator free days in the lower tidal volume group.
PEEP
It was postulated that PEEP may be beneficial in ARDS as it reduces biotrauma, maintains the patency of injured alveoli, reduces intrapulmonary shunting and improves ventilation-perfusion mismatch. However evidence regarding its use is inconclusive. Numerous large centre trials have demonstrated no difference in outcome or mortality between patients ventilated with lower PEEP vs. higher PEEP (8 vs. 14 cm H₂O).
15 16 17 Yet a recent JAMA systematic review and meta-analysis showed that although higher PEEP ventilation was not associated with improved hospital survival, it was associated with improved survival among the ARDS subgroup of ALI and suggested that an optimal level of PEEP remains unestablished but may be beneficial.
18
ECMO (Extracorporeal membrane oxygenation)
This is a modified longer term form of cardiopulmonary bypass which aims to provide gas exchange across an artificial membrane external to the body, allowing the lungs time to recover. It is confined to a few specialist centres in the UK and the first results from the CESAR multicentre randomised controlled trial were published in the Lancet in 2009. It showed improved survival in adult patients with severe but potentially reversible respiratory failure on ECMO, as compared to conventional ventilation and demonstrated cost effectiveness in settings like the UK healthcare system.
19 This therefore may be a treatment strategy to consider in extreme cases resistant to conventional therapy.
OTHER STRATEGIES
A current meta-analysis looking at prone positioning concluded that randomised controlled trials failed to demonstrate improved outcomes in ARDS patients overall. There is a decrease in absolute mortality in severely hypoxaemic patients with ARDS but as long term proning can expose ALI patients to unnecessary complications, it should only be used as rescue therapy for individuals resistant to conventional treatment.
20No evidence supporting specific weaning programmes exists and a recent Cochrane review showed no evidence to support recruitment manoeuvres in ALI.
21
Therefore the aim of ventilation is low volumes with permissive hypercapnia, providing adequate oxygenation (regarded as a partial pressure of arterial oxygen >8kPa) whilst trying to avoid oxygen toxicity lung injury.
Fluid Management
Fluid management has to balance the need for enough fluid to maintain an adequate cardiac output and end organ perfusion, with a low enough intravascular pressure to prevent high capillary hydrostatic pressures, which could cause pulmonary oedema, worsen oxygen uptake and carbon dioxide excretion. Evidence supports a negative fluid balance in patients not requiring fluid for shock.
Studies as early as 1990 showed a reduction in pulmonary wedge pressure was associated with increased survival
22 and extravascular lung water was associated with poor outcomes
23 in ARDS patients.
The ARDSnet FACTT study looked at two fluid regimens comparing liberal fluid management (a net gain of approximately 1 litre per day) with a conservative fluid management (zero net gain over first seven days).
24 Although there was no significant difference in (the) primary outcome of 60 day mortality, the conservative management group had improved lung function, shortened duration of mechanical ventilation and intensive care and had no increased incidence of shock or use of renal replacement. This is supported by a recent retrospective review, which concluded negative cumulative fluid balance at day 4 of acute lung injury is associated with significantly lower mortality, independent of other measures of severity of illness.
25
Pharmacotherapy
To date no pharmacological agent has been demonstrated to reduce mortality among patients with ALI.
26 However ALI encompasses a wide range of patients with varying aetiology and comorbidities. It may be that on subdividing ALI patients, some therapies may be suitable for specific circumstances but at present there is little literature to support this.
EXOGENOUS SURFACTANT
Since the 1980’s numerous randomised controlled trials have demonstrated no benefit from synthetic, natural or recombinant surfactant use in adults with ALI.
INHALED NITRIC OXIDE
Despite providing selective vasodilatation and improving ventilation perfusion mismatch, trials have only showed short lived improvement in oxygenation and no change in mortality with nitric oxide use. At present it plays no role in standard ALI treatment and should be reserved for rescue therapy in patients difficult to oxygenate.
27
STEROIDS
Despite the potential for steroids to benefit ALI patients due to anti-inflammatory properties, clinical trials demonstrate no improved mortality when given early or late in disease progression and given concerns regarding their role in development of neuromuscular disorders associated with critical illness, a recent large randomised controlled trial argued against steroid use in ALI.
28
INTRAVENOUS SALBUTAMOL
Beta 2 agonists were shown to be experimentally beneficial in ALI due to increasing fluid clearance from alveolar space, anti-inflammatory properties and bronchodilation.
29 The BALTI trial published in 2006, investigated the effects of intravenous salbutamol in patients with ARDS. It showed decreased lung water at day 7, lowered Murray lung injury scores and lower end expiratory plateau pressures but an increase in incidence of supraventricular tachycardias and therefore further investigation is needed before it can be recommended as treatment for ALI.
30 The BALTI-2 trial is currently underway in the UK, to further assess possible benefits and complications.
Other new and promising treatments which are currently being evaluated in trials are activated protein C and granulocyte-macrophage colony-stimulating factor (GM-CSF).
MORTALITY
Mortality rates of patients with ALI and ARDS are similar, with both being around 35-40%.³ Controversy exists regarding whether mortality rates in ALI are decreasing,
31 or have stayed static.
32 Nonetheless death in patients with ALI is rarely from unsupportable hypoxaemic respiratory failure but from complications of the underlying predisposing conditions or multiple organ failure.
33
There is some evidence related to racial and gender differences in mortality (worse in African Americans and males)
34 and that thin patients have increased mortality and obese patients have somewhat lower mortality than normal weight individuals
35 but the main independent risk factors for increased mortality are shown in Table 5.
Table 5 Independent risk factors for increased mortality in ALI as identified in multicentre epidemiological cohorts
|
· Old age
· Worse physiological severity of illness
· Shock, on admission to hospital
· Shorter stay in the ICU after ALI onset
· Longer hospital stay before ALI onset
· Increased opacity on chest radiography
· Immunosupression
|
OUTCOMES
Long term problems are related to neuromuscular, neurocognitive and psychological dysfunction rather than pulmonary dysfunction. (Table 6) There is poor understanding of the mechanisms which cause these sequelae and therefore prevention of these outcomes and planning rehabilitation can be difficult.
Table 6 Long Term Outcomes in ARDS survivors and caregivers
|
Neuromuscular dysfunction
· critical illness polyneuropathy
· critical illness myopathy
· entrapment neuropathy
Neurocognitive dysfunction involving
· memory
· executive function
· attention
· concentration
Psychological dysfunction
· Post traumatic stress disorder
· Depression
· Anxiety
Other
· Pulmonary dysfunction
· Tracheostomy site complications
· Striae
· Frozen joints
Caregiver and financial burden
|
A recent study into patients who survived ALI showed they require support during discharge from ICU to other hospital settings and again once in the community regarding guidance on home care, secondary prevention and support groups.
36
CONCLUSION
The syndrome which encompasses ALI and ARDS is common and under-recognised, with many clinicians encountering it outside the ICU setting. Despite advances in identification and management, morbidity and mortality is still high. Care should focus on supportive treatment and managing the underlying cause, whilst specifically aiming for low volume ventilation and conservative fluid balance. Ongoing research is still needed to hone the diagnostic criteria, define genetic risk factors and develop new treatment strategies to improve outcome. The new challenge for clinicians is how to address the long term outcomes of survivors and their relatives which will be an increasingly important problem in the future.²⁶
Competing Interests
None Declared
Author Details
HELEN LAYCOCK, MBBS BSc (Hons), ST3 Anaesthetics Watford Hospital.
ABID RAJAH, MB ChB FRCA, FFARCSI, Consultant in Anaesthesia and Intensive Care, Watford Hospital.
CORRESPONDENCE: ABID RAJAH, Consultant in Anaesthesia and Intensive Care, Watford Hospital.
Email: ARajah@aol.com |
References
1. Ashbaugh DG, Bigelow DB, Petty TL et al. Acute Respiratory Distress in Adults. Lancet. 1967; 2: 319-3232. Murray JF, Matthay MA, Luce JM et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988; 138: 720-7233. Bernard GR, Artigas A, Brigham KL et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994 Mar; 149 (3 Pt 1): 818-8244. Rubenfeld GD, Herridge MS. Epidemiology and Outcomes of Acute Lung Injury. Chest. 2007; 131 (2): 554-5625. McCallum NS, Evans TW. Epidemiology of Acute Lung Injury. Current Opinion in Critical Care. 2005: 11; 43-496. Rubenfeld GD, Caldwell E, Peabody E et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685-16937. Finney SJ, Evans TW. Acute lung injury outside the ICU: a significant problem. Critical Care. 2007; 11: 1698. Hudson LD, Milberg JA, Anardi D et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:293-301.9. Kamp R, Sun X, Garcia JG. Making genomics functional: deciphering the genetics of acute lung injury. Proc Am Thorac Soci. 2008; 5: 348-5310. Moss M, Guidot DM, Stienberg KP et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Critical Care Medicine 2000; 28:2187-219211. Meduri GU. Late adult respiratory distress syndrome. New Horiz 1993; 1:563-57712. Rocco PRM, Santos CD, Pelosi P. Lung parenchyma remodelling in acute respiratory distress syndrome. Minerva Anestesiologica. 2009; 75: 730-74013. Gattinoni L, Pesenti A. The concept of “baby lung” Intensive Care Med 2005; 31: 776-78414. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301-130815. The Acute Respiratory Distress Syndrome Network. Higher versus lower positive end-expiratory pressures in patients with acute respiratory distress syndrome. N Engl J Med. 2004; 351: 327-33616. Meade MO, Cook DJ, Guyatt GH et al. Ventilation strategy using low tidal volumes, recruitment manoeuvres and high positive end expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomised controlled trial. J Am Med Assoc; 2008: 299: 637-64517. Mercat A, Richard J-CM, Vielle B. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomised controlled trial. J Am Med Assoc; 2008: 299: 646-65518. Briel M, Meade M, Mercat A et al. Higher vs. lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA; Mar 2010: 303(9):865-87319. Peek GJ, Mugford M, Tiruvoipati R et al. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet; Oct 2009: 374(9698):1351-136320. Gattinoni L, Carlesso E, Taccone P et al. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010; 76: 448-45421. Hodgson C, Keating JL, Holland AE et al Recruitment manoeuvres for adults with acute lung injury receiving mechanical ventilation. Cochrane Database Syst Rev; 2009 Apr; 15; (2):CD00666722. Humphrey H, Hall J, Sznajder I, et al. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990; 97: 1176-118023. Sakka SG, Klein M, Reinhart K, et al. Prognostic value of extravascular lung water in critically ill patients. Chest 2002; 122: 2080-208624. The Acute Respiratory Distress Syndrome Network. Comparison of Two Fluid Management Strategies in Acute Lung Injury. New Engl J Med. 2006; 354: 2564-257525. Rosenber AL, Derchert RE, Park PK et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. Journal of Intensive Care Medicine. 2009; 24(1): 35-4626. Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006; 21: 119-14327. Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest 2007; 131(3): 913-92028. The Acute Respiratory Distress Syndrome Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671-168429. Groshaus HE, Manocha S, Walley KR et al. Science review: Mechanisms of beta-receptor stimulation-induced improvement of acute lung injury and pulmonary oedema. Critical Care 2004; 8 (4): 234-24230. Perkins GD, McAuley DF, Thickett DR et al. The ß-Agonist Lung Injury Trial (BALTI): A Randomized Placebo-controlled Clinical Trial Am J Respir Crit Care Med. 2006; 173:281-28731. Herridge MS, Angus DC. Acute Lung Injury – affecting many lives. N Engl J Med 2005; 353: 1736-173832. Phua J, Badia JR, Adhikari NK et al. Has mortality from acute respiratory distress syndrome decreased over time?: a systematic review. Am J Respir Crit Care Med. 2009 Feb; 179(3): 220-22733. Stapleton RD, Wang BM, Hudson LD et al. Causing and timing of death in patients with ARDS. Chest 2005; 128: 525-53234. Moss M, Manniono DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple cause mortality data (1979-1996) Critical Care Medicine 2002; 30: 1679-168535. O’Brien JM Jr, Phillips GS, Ali NA et al. Body Mass Index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Critical Care Medicine 2006; 34: 738-74436. Lee CM, Herridge MS, Matte A et al. Education and support needs during recovery in acute respiratory distress syndrome survivors. Critical Care 2009; 13(5):R153

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.







