Community-acquired urinary tract infection in the elderly
Mahesh E, Medha Y, Indumathi V A, Prithvi S Kumar, Mohammed Wasim Khan and Punith K
Cite this article as: BJMP 2011;4(1):a406
|
|
Abstract Background: Urinary tract infection (UTI) in the elderly poses a very serious problem. The knowledge of microbiology and antibiotic susceptibility of micro-organisms causing the disease is vital for defining the empirical treatment. There are no large-scale studies on the same from India. Aim: To find out the common presenting symptomatology and risk factors associated with UTI and distribution of isolated uropathogens and their resistance pattern. Settings and design: Prospective study done in a tertiary care centre in Bangalore. Methods and material: The study included elderly patients aged 65 years and above who were admitted, or visited the outpatient departments in the hospital, and had confirmed UTI. Results and conclusions: Fever (57/194 - 29.4%) and dysuria (52/194 - 26.8%) were the most common symptoms of UTI. Diabetes Mellitus (DM) was the most common risk factor associated with UTI. Extended Spectrum Beta-Lactamase (ESBL) producing Escherichia coli (E.coli) (93/194 - 47.94%) was the most commonly isolated pathogen. Of the total, 56.2% of the uropathogens showed ESBL positivity. Overall, the highest antibiotic resistance was recorded for Fluoroquinolones (79.9%). Keywords: Uropathogen, Elderly, Antibiotic Resistance, ESBL |
Introduction
Urinary tract infection (UTI) is the second most common infectious complaint in geriatric clinics overall, and the most common outpatient complaint caused by bacteria.1 The diagnosis and treatment of UTI in the elderly is not the same as treating UTI in adults. In frail elderly patients with age-associated multiple severe underlying disorders and cognitive impairment, early recognition of bacteraemic UTI and prompt, appropriate treatment are critical in reducing the mortality.2Also, the extensive and inappropriate use of antimicrobial agents has invariably resulted in the development of antibiotic resistance which, in recent years, has become a major problem worldwide.3 The diagnosis and empirical treatment of UTI in the elderly is challenging and a sound knowledge of the prevalent epidemiology of bacteria and their resistance pattern is necessary for the same. However, there is not much information on the aetiology and resistance pattern of UTI in the elderly in India. This study was done to find out the present uropathogen profile causing UTI in our centre and their antibiotic resistance patterns.
Subjects and methods
This prospective study was done at our tertiary care centre from January to December 2008. The study included all patients who were admitted or visited the outpatient departments in the hospital with symptoms of UTI during the study period and had UTI confirmed by positive urine culture reports. Only one sample from each subject was considered. Subjects with clinical symptoms of UTI but no growth on culture were excluded from final analysis. Subjects who were treated with another antimicrobial within the previous 48 hours, or within 24 hours if only a single dose and in the presence of an appropriate positive culture and ileal loops or vesicoureteral reflux were also excluded from the study. Complete data regarding demography, sex preponderance, associated symptoms, pathogenic organisms causing UTI and their antibiotic resistance were collected.
Overall, 194 subjects were included in the study (male: 116, female: 78). The mean age of the study population was 73.54±7.19 years, ranging between the ages of 65 and 96. The distribution of patients according to gender across various age groups is given in table 1. A general trend of more male subject enrolment was seen across all the age groups.
|
Age group |
Male |
Percent |
Female |
Percent |
Total |
Percent |
|
65-74 |
66 |
56.9 |
48 |
61.5 |
114 |
58.8 |
|
75-84 |
40 |
34.5 |
24 |
30.8 |
64 |
33.0 |
|
85-94 |
10 |
8.6 |
5 |
6.4 |
15 |
7.7 |
|
≥95 |
0 |
0 |
1 |
1.3 |
1 |
.5 |
|
Total |
116 |
100.0 |
78 |
100.0 |
194 |
100.0 |
Isolation and identification of uropathogens
A clean catch midstream specimen, or suprapubic aspirate in subjects who were unable to give the former, was collected in a sterile, wide-mouth, leak-proof container to hold approximately 50ml from these subjects. Using a calibrated loop method of loop diameter 4 mm, 10 µL of the uncentrifuged specimen was transferred onto the agar plate and streak, using the modified Mayo’s technique without flaming the loop for isolation, and incubated at 35- 370C for 24 hours. A specimen was considered positive for UTI if a single organism was cultured at a concentration of >105 Colony Forming Units/ml. The Gram-positive and Gram-negative organisms culture isolates were further identified by using various biochemical reactions up to genus/species level wherever applicable.
Antibiotic sensitivity testing
In the presence of any potential growth, antibiotic sensitivity testing was done by the Modified Kirby-Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.4 The antibiotics tested were Imepenem, Meropenem, Ciprofloxacin, Ofloxacin, Norfloxacin, Amikacin, Gentamicin, Nitrofurantoin and Cotrimoxazole (Pathoteq Labs, India).
Extended Spectrum Beta-Lactamase (ESBL) detection
The screening for ESBL was done using Cefpodoxime (<17mm), Ceftazidime (<22mm), Aztreonam (<27mm), Cefotaxime (<27mm) and Ceftriaxone (<25mm). If the organisms showed the zone of inhibition lower than the minimum for any antibiotic disc, ESBL positivity was suspected. The phenotypic confirmation was done by testing the strain against Ceftazidime (Ca) and Ceftazidime/Clavulanic Acid. A > 5mm diameter of the zone of inhibition for Ceftazidime/Clavulanic Acid in comparison to Ceftazidime was considered indicative of ESBL production. Escherichia coli (E. coli) ATCC 25922 was used as ESBL negative and Klebsiella pneumoniae (K. pneumoniae) 700603 was used as ESBL positive reference strain.4
Statistical analysis
Descriptive statistics (totals, means, percentages, and standard deviations) were conducted using the statistical software package - SPSS Version 16.0 (SPSS Inc., Chicago, USA). Age, gender, organisms causing UTI, their antibiotic sensitivity and resistance, symptomatology of these subjects, and risk factors for UTI were included in the analysis and the results presented in tables and figures.
Results
Fever (57/194 - 29.4%) and dysuria (52/194 - 26.8%) were common symptoms of most UTI patients (Fig. 1). Diabetes mellitus (DM) and recent uro-genital instrumentation were the most common risk factors associated with UTI in the present study (Table 2). The organism profile and their antibiotic resistance profile were similar in patients with or without DM.
Figure 1. Various symptomatologies seen in patients with urinary tract infection during the initial presentation
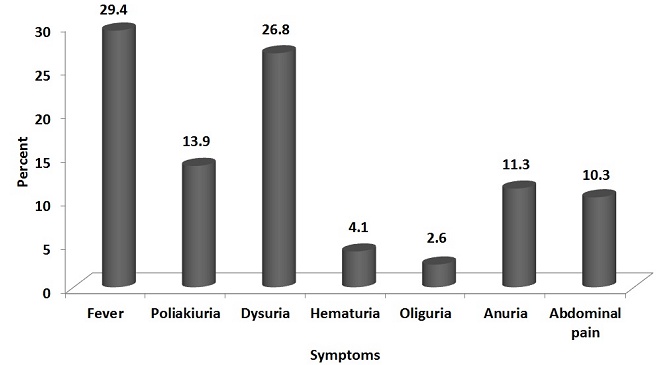
Table 2. Frequency of various risk factors in subjects with urinary tract infection.
|
Risk Factor |
Frequency |
Percent |
|
Catheterization |
29 |
14.9 |
|
Diabetes Mellitus |
97 |
50.0 |
|
Immunosuppression |
2 |
1.0 |
|
Recent history of uro-genital Instrumentation |
43 |
22.2 |
|
Recurrent urinary tract infection |
14 |
7.2 |
|
Renal stones |
5 |
2.6 |
E. coli (138/194 - 71.1%) was the most commonly isolated pathogen responsible for UTI in the present study (Figure 2). 56.2% of the total infection was caused by ESBL positive organisms. The antimicrobial potency and spectrum for nine selected antimicrobial agents (Imepenem, Meropenem, Ciprofloxacin, Norfloxacin, Ofloxacin, Gentamicin, Amikacin, Nitrofurantoin and Cotrimoxazole) against the uropathogens were studied. The highest and least antibiotic resistance was noted against fluoroquinolones (79.9%) and carbapenems (3.61%) respectively (Fig. 3).
Figure 2. Frequency and distribution pattern of urinary tract infection pathogens and percentage Extended Spectrum Beta-Lactamase (ESBL) production.

Figure 3. Resistance pattern to various antibiotics of the uropathogens

Discussion
While increased frequency and dysuria are usual symptoms of UTI, uncertainty looms around the same as these symptoms can be masked by catheterisation, or be common and chronic in the elderly even in the absence of UTI.5-10Fever was the most common symptom of UTI in the present study as with similar studies worldwide.11-13 Studies have found that the elderly do not lack a febrile response; that an elevated temperature was the most common initial symptom, a marker for a serious infection, and the most important clinical indicator for antibiotic treatment.14-16 Whitelaw et al17 reported that a delay in interpreting fever as a symptom of UTI led to a high mortality rate in the elderly within 24 hours of admission.
Diabetes isconsidered as an important risk factor for UTI with manyauthors having defined UTI in patients with DM as complicated when the UTI is symptomatic.18-19 However, the authors did not find that DM influenced the organism profile and their antibiotic resistance in the present study. Bonadio et al20 studied the influence of DM on the spectrum of uropathogens and antimicrobial resistance in elderly adult patients with asymptomatic UTI (mostly hospital-acquired). They found that DM per se did not seem to influence the isolation rate of different uropathogens and their susceptibility patterns to antimicrobials. These findings indicate that, although DM is a known immunomodulator, the role played by the same in altering the antibiotic resistance is minimal compared to recent invasive procedures.
Although the uropathogen profile of the present study resembles similar studies worldwide, the antibiotic resistance of these organisms was unusually high.2, 21 Cotrimoxazole is the recommended drug for treating UTI. However, more than one third of the study subjects were resistant to the first-line drug. 79.9% of the uropathogens were resistant to fluoroquinolones, which are considered as the second-line drug. As prior fluoroquinolone use is a known risk factor for fluoroquinolone-resistantE. coli infection, it is plausible that frequent fluoroquinolone prescriptions may be contributing to the observed resistance.22-23 Aypak et al 24 found that treatment durations were statistically longer than the recommended three-day course when patients were empirically treated with fluoroquinolones due to increased resistance rates, and suggested to discourage the empirical use of fluoroquinolones in UTI.
The most troublesome finding of the present study is that ESBL-positive organisms accounted for 56.2% of the total infection. Not much information on ESBL-producing organisms causing UTI is available from India and most of these reports are from the younger population. The prevalence of ESBL-positive UTI in these studies varied between 26.6% and 48.3%.25-26 To the best of our knowledge, this is the highest ever reported prevalence of ESBL-positive UTI in the elderly worldwide. ESBL-producing organisms are frequently resistant to many of the antimicrobial agents usually recommended for the treatment. As lesser new antibiotics are available for their management, we need to be concerned of this issue in years to come especially in tertiary care centres. A unified antibiotic protocol is necessary to limit the morbidity and mortality associated with inappropriate and under-treatment of UTI.
The limitations of the present study were that altered mental status was not considered as one of the clinical manifestations of UTI in the elderly, which could have mitigated the total number of study subjects included in the study. In addition, the phenotypic confirmation of ESBL-positive organisms was done using only Ceftazidime/Clavulanic Acid and not Cefotaxime/Clavulanic Acid as per the latest CLSI guidelines. As a result, there may be under-reporting of the incidence of ESBL organisms in the present study.
In conclusion, we report a significantly high resistance to common antibiotics among the uropathogens in the present study. Furthermore, the very high rate of ESBL-positive UTI is of concern, and monitoring for the same is necessary to prevent treatment failure and increased morbidity and mortality with UTI.
|
Competing Interests None declared Author Details MAHESH E, Associate Professor, Department Of Nephrology, M S Ramaiah Medical College MEDHA Y, Professor And Head, Department Of Medicine, M S Ramaiah Medical College INDUMATHI V A, Consultant Microbiologist, Gokula Metropolis Clinical Labs, M S Ramaiah Medical College PRITHVI S KUMAR, MOHAMMED WASIM KHAN, PUNITH K, Residents, M S Ramaiah Medical College CORRESPONDENCE: Punith K, Address: No. 28/18, 19th Main Road, MC Layout, Vijaynagar, Bangalore-560040, India Email: drpunith@gmail.com |
References
- O'Donnell J, Gelone S, Abrutyn E. Selecting drug regimens for urinary tract infection: current recommendations. Infect Med 2002;19:14-22.
- Tal S, Guller V, Levi S, Bardenstein R, Berger D, Gurevich I et al. Profile and prognosis of febrile elderly patients with bacteremic urinary tract infection. J Infect 2005;50:296-305.
- Goldstein FW. Antibiotic susceptibility of bacterial strains isolated from patients with community-acquired urinary tract infections in France. Multicentre Study Group. Eur J Clin Microbiol Infect Dis 2000;19:112-7.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA, 2006.
- Nicolle L. Urinary tract infection in the elderly.J Antimicrob Chemother 1994;33: 99-109.
- Fune L, Shua-Haim J, Ross J, Frank E. Infectious diseases in the elderly. Clinical Geriatrics 1998;6:31-50.
- Beier MT. Management of Urinary tract infections in the nursing home elderly: a proposed algorithmic approach. Int J Antimicrob Agents 1999;11:275-84.
- 8.Nicolle LE; SHEA Long-Term-Care-Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol 2001;22:167-75.
- 9.Baldassarre JS, Kaye D. Special problems of urinary tract infection in the elderly. Med Clin North Am 1991;75:375-90.
- Rudman D, Hontanosas A, Cohen Z, Mattson DE.Clinical correlates of bacteremia in a Veterans Administration extended care facility. J Am Geriatr Soc 1988;36:726-32.
- Meyers BR, Sherman E, Mendelson MH, Velasquez G, Srulevitch-Chin E, Hubbard M, Hirschman SZ. Bloodstream infections in the elderly. Am J Med1989;86:379-84.
- Richardson JP, Hricz L. Risk factors for the development of bacteremia in nursing home patients. Arch Fam Med1995;4:785-9.
- Chassagne P, Perol MB, Doucet J, Trivalle C, Ménard JF, Manchon ND et al. Is presentation of bacteremia in the elderly the same as in younger patients? Am J Med 1996;100:65-70.
- Katz PR, Beam TR Jr, Brand F, Boyce K. Antibiotic use in the nursing home. Physician practice patterns. Arch Intern Med 1990;150:1465-8.
- Yoshikawa TT, Norman DC. Approach to fever and infection in the nursing home. J Am Geriatr Soc 1996;44:74-82.
- Alessi CA, Harker JO. A prospective study of acute illness in the nursing home. Aging (Milano) 1998;10:479-89.
- Whitelaw DA, Rayner BL, Willcox PA. Community-acquired bacteremia in the elderly: a prospective study of 121 cases. J Am Geriatr Soc. 1992 Oct;40(10):996-1000
- Stapleton A. Urinary tract infections in patients with diabetes. Am J Med. 2002 Jul 8;113 Suppl 1A:80S-84S
- Ronald A, Harding G. Complicated urinary tract infections. Infect Dis Clin North Am 1997;11:583-592.
- Bonadio, M., Costarelli, S., Morelli, G., Tartaglia, T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection. BMC Infect Dis 2006;6:54.
- Ackermann RJ, Monroe PW. Bacteremic urinary tract infection in older people. J Am Geriatr Soc 1996;44:927-33.
- Cohen AE, Lautenbach E, Morales KH, Linkin DR. Fluoroquinolone-resistant Escherichia coli in the long-term care setting. Am J Med 2006;119:958-63
- Das, R., Perrelli, E., Towle, V., Van Ness PH., Juthani-Mehta, M. Antimicrobial Susceptibility of Bacteria Isolated from Urine Samples Obtained from Nursing Home Residents. Infect Control Hosp Epidemiol 2009;30: 1116-9.
- Aypak, C., Altunsoy, A., Düzgün, N. Empiric antibiotic therapy in acute uncomplicated urinary tract infections and fluoroquinolone resistance: a prospective observational study. Ann Clin Microbiol Antimicrob 2009;8:27.
- Khurana S, Taneja N, Sharma M. Extended spectrum beta-lactamase mediated resistance in urinary tract isolates of family Enterobacteriaceae. Indian J Med Res 2002;116:145-9.
- Tankhiwale SS, Jalgaonkar SV, Ahamad S, Hassani U. Evaluation of extended spectrum beta lactamase in urinary isolates. Indian J Med Res 2004;120:553-6.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




