Endobronchial Ultrasound Transbronchial Needle Aspiration (EBUS-TBNA) in the Diagnosis of Lung Cancer & Mediastinal/ Hilar Lymphadenopathy
Amer Saleem, Neal Navani & Vivek Vohra
Cite this article as: BJMP 2019;12(3):a025
|
|
Abstract Background: EBUS-TBNA is an out-patient minimally invasive procedure of choice to explore mediastinal/hilar lymph nodes & lesions close to the tracheobronchial tree. In the UK there has been a significant growth in this area over the last 10 years. Abbreviations: EBUS - endobronchial ultrasound, TBNA - transbronchial needle aspiration, GI - gastrointestinal, IMHL - Isolated Mediastinal & Hilar Lymphadenopathy. |
Introduction
Convex probe EBUS-TBNA has been a major development in respiratory medicine. In the last decade we have seen numerous articles supporting the high diagnostic accuracy of EBUS-TBNA in the diagnosis of lung cancer, staging of lung cancer, diagnosis of extra-thoracic malignancies & benign conditions (e.g., TB & sarcoidosis)1. Patients included in this study reflect the real-life referrals that we see as respiratory physicians in our daily practice. This shows that the trend of doing EBUS-TBNAs for non-cancer patients is rising. Lung cancer is a common cause of cancer death worldwide2. Various guidelines (including NICE) have found this procedure safe & recommend it for the staging of lung cancer. In the last 10 years, lots of district general hospitals have started this service in UK & it is mainly delivered by respiratory physicians.
This has provided a specialist service for patients in their local area, which has reduced travelling and waiting times.
Setting & Methods
In this district general hospital under discussion, EBUS service was setup in 2018, under supervision of a tertiary care centre. We carried out 82 procedures during the first year of this service. All of these cases were reviewed for this article. Data was recorded on an excel spreadsheet (data included: number of cases, age, gender, lymph node stations sampled, complications, pathology & microbiology results of EBUS TBNA). Minimum of 4 passes were done at each lymph node station. Where EBUS was done for diagnostic purposes, stations to be sampled were at the discretion of the operator. Samples obtained via EBUS-TBNA were flushed into CytoLyt (methanol-water solution). EBUS-TBNAs were carried out in the absence of rapid on-site evaluation (ROSE). Where a cancer was suspected but EBUS-TBNA showed normal findings, samples were obtained via another modalities (e.g., CT biopsy) & FDG PET was carried out as well (if not done already). In cases of isolated mediastinal & hilar lymphadenopathy (IMHL), where EBUS-TBNA did not reveal any pathology, interval surveillance CTs were carried out for monitoring purposes. Where lymphadenopathy did not resolve, surveillance scans were carried out for a year. The outcomes of these surveillance CTs & PET CTs were also reviewed for this study. Diagnosis of reactive lymphadenopathy was made if EBUS-TBNA sample did not reveal any pathology, repeat CT did not show any change (or showed reduction/ resolution of lymphadenopathy) & the clinician did not consider the patient to have another diagnosis. EBUS-TBNA was labelled as false negative, if pathology result was negative, but node was positive on PET (in suspected cancer patients).
Results
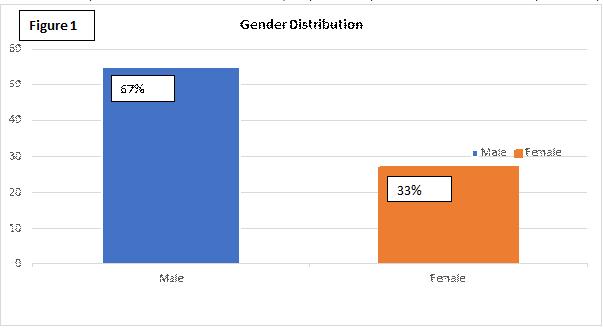
Out of these 82 patients who underwent EBUS-TBNA, 55 (about 67%) were male & 27 were female (about 33%) (Figure 1).
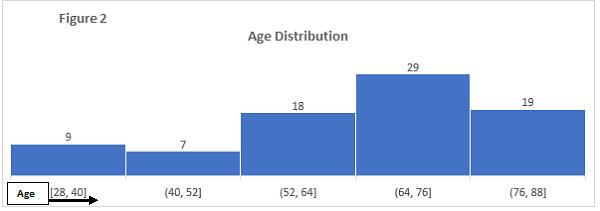
The age range of patients at the time of procedure was 28 to 88 years. Majority of the patients were between the age of 52 – 88 years (80% of the cases) (Figure 2).
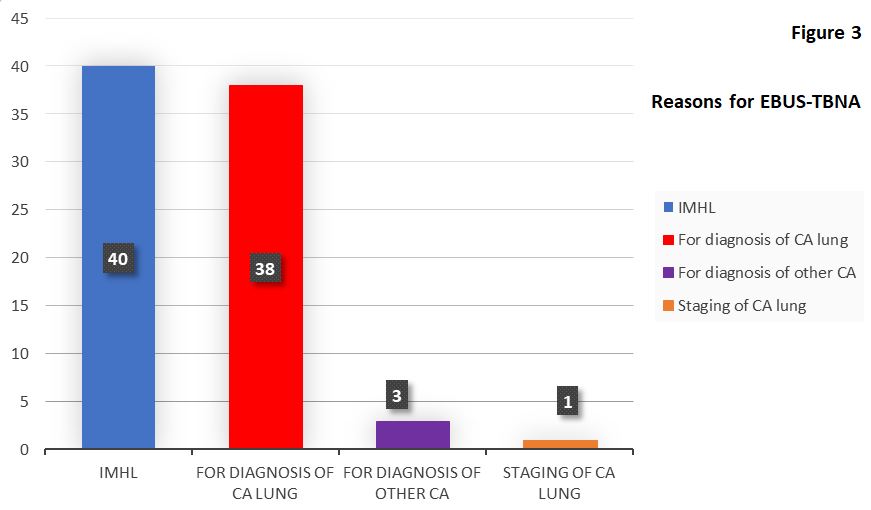
The 82 EBUS-TBNA procedures were carried out for the following main reasons (Figure 3):
A. 42 procedures for cancer reasons (i.e. 51% of the total procedures)
a. For diagnosis of lung cancer (38 procedures)
b. Diagnosis of suspected extra-thoracic cancer (3 cases)
c. Staging of lung cancer (1 case)
B. 40 procedures for IMHL (i.e. 49%)
The final diagnoses in 38 procedures carried out for “diagnosis of lung cancer” were as follows:
1. 25 patients were diagnosed with lung cancer (12 squamous cell cancers, 7 adenocarcinomas, 4 small cell cancers, 1 undifferentiated lung cancer & 1 neuroendocrine tumour)
2. Final diagnosis in 9 cases was reactive lymphadenopathy (repeat CT showed resolution of lymph nodes in 3 cases, reduction in the size in 1 case & stable nodes in 5 cases)
3. Extra-thoracic malignancies were diagnosed in 2 cases (1 metastatic prostate cancer & 2nd was metastatic disease from primary parotid gland tumour)
4. We had false negative results in 2 cases (1 patient was diagnosed with small cell lung cancer on CT biopsy & 2nd with adenocarcinoma on ultrasound biopsy)
It was found in 11 cases, where the clinicians initial suspicion was a possible lung cancer, that the final diagnoses were reactive lymphadenopathy and extra-thoracic malignancies.
Some of these patients had lung nodules as well (along with mediastinal & hilar lymphadenopathy). These nodules either resolved or remained stable. In the case of metastatic prostate cancer, prior MRI showed prostate confined disease & the clinician suspected this size significant lymphadenopathy to be due to a lung primary. In the case of metastatic parotid tumour, the initial diagnosis of parotid cancer was a very long time ago & metastatic disease was not expected.
Final diagnoses in 3 patients who had EBUS-TBNA for “extra-thoracic malignancies” were as follows:
1. Prostate cancer (here pelvic MRI showed locally advanced disease)
2. Colon cancer (known colon cancer)
3. Ovarian cancer (patient had ovarian mass & abdominal/pelvic lymphadenopathy)
As most of the surgical patients go directly to tertiary care centres (from this hospital), we therefore didn’t have many patients for staging purposes during the 1st year of the service. We only had 1 patient for “staging EBUS-TBNA” during this time. In this case stations 4L, 7 & 12L were sampled. Only station 12L was PET positive & also positive on EBUS-TBNA sample. Station 4L & 7 were PET and EBUS-TBNA negative. There was no size significant nodes seen on staging CT in any other area, only 12L node was PET avid & we were not able to identify any size significant lymphadenopathy at any other station via EBUS as well. Sensitivity in this staging EBUS was 100%.
In these 42 diagnostic & staging procedures (carried out for cancers or suspected cancers), summary of the pathological diagnoses from lymph nodes aspirates is as follows:
1. Squamous cell carcinoma of lung 13 approximately (31%)
2. Adenocarcinoma of lung origin 7 approximately (17%)
3. Small cell lung cancer 4 approximately (9.5%)
4. Neuroendocrine tumour of lung origin 1 approximately (2.3%)
5. Undifferentiated lung cancer 1 approximately (2.3%)
6. Metastatic prostate cancer 2 approximately (4.75%)
7. Metastatic parotid gland cancer 1 approximately (2.3%)
8. Metastatic ovarian cancer 1 approximately (2.3%)
9. Metastatic colon cancer 1 approximately (2.3%)
10. False negative 2 approximately (4.75%)
11. Reactive lymphadenopathy 9 approximately (21.5%)
Out of the 40 procedures for IMHL, we were not able to get an adequate sample in 1 case and this patient underwent repeat EBUS-TBNA. Repeat sample showed granulomas; findings were consistent with the clinical diagnosis of sarcoidosis. Final diagnoses in these 40 cases are as follows:
1. Metastatic adenocarcinoma from pancreaticobiliary origin = 1 (2.5%)
2. Bronchogenic cyst = 1 (2.5%)
3. Insufficient sample = 1 (2.5%)
4. Tuberculosis = 3 (7.5%)
5. Granulomas = 16 (40%)
6. Reactive lymphadenopathy = 18 (45%)
Serious diagnoses were made in 10% cases of IMHL (4 out of 40). One patient had metastatic adenocarcinoma from pancreaticobiliary origin & didn’t have any abdominal symptoms or any abnormalities on CTs in the abdomen. 3 patients were diagnosed & later treated for active tuberculosis. Out of these 3 patients only 1 had features of active disease, but sputum negative. The other 2 patients had only mediastinal lymphadenopathy, no lung infiltrates & no sputum production.
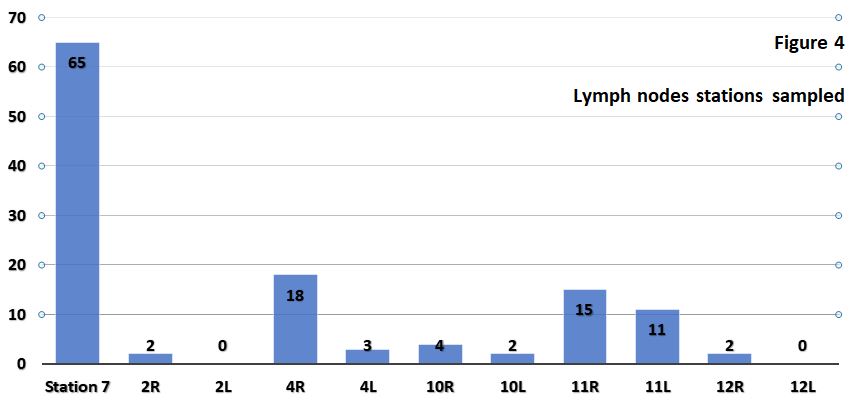
A total of 122 lymph nodes were sampled. Details are as follows (figure 4):
| Lymph node station | Times sampled | % |
| Station 7 | 65 | 53.3 |
| 4R | 18 | 14.8 |
| 11R | 15 | 12.3 |
| 11L | 11 | 9 |
| 10R | 4 | 3.2 |
| 4L | 3 | 2.5 |
| 2R | 2 | 1.6 |
| 10L | 2 | 1.6 |
| 12R | 2 | 1.6 |
| 2L | 0 | 0 |
| 12L | 0 | 0 |
Commonly sampled nodes were station 7 nodes. This is consistent with international literature published on EBUS-TBNA.
There were no complications from the procedures performed. None of our patients experienced significant airway bleeding (requiring admission or blood transfusion), mediastinal infection, pneumothorax, pneumo-mediastinum, haemo-mediastinum or airway lacerations.
Discussion
EBUS TBNA is one of the methods to access the mediastinal & hilar lymph nodes. This is a minimally invasive way to get samples from these nodes. Several invasive, minimally-invasive & non-invasive techniques are available to diagnose & stage lung cancers. Choice depends upon the extent of the disease. About 50% of lung cancer patients have evidence of metastatic disease at the time of presentation 3. Patients with intrathoracic disease undergo several investigations. Now we know that EBUS-TBNA should be considered as the initial investigation for patients with early stage suspected lung cancer 4. Research carried out has shown that EBUS-TBNA had a sensitivity of 90% 5. A recent national BTS audit on bronchoscopy & EBUS showed national diagnostic sensitivity of 90% for staging EBUS-TBNA. BTS quality standards statement sets target of 88% sensitivity for staging EBUS-TBNA6. As far as diagnostic EBUS-TBNA is concerned, we had 2 false negative results out of 41 (4.8%), that gives the sensitivity for diagnostic procedures of 95.2%.
There is significant evidence available that ROSE does not increase the diagnostic yield of even conventional TBNA 7. Trisolini et al demonstrated in this randomised controlled trial that ROSE did not give any significant diagnostic advantage & did not affect the percentage of adequate specimens. Articles have also shown that ROSE does not reduce the EBUS-TBNA procedure time 8. The use of immunohistochemistry on EBUS-TBNA reduces the rate of unclassified non-small cell lung cancer when compared with cytological diagnosis alone 9. EBUS-TBNA samples are sufficient to allow immunohistochemical and molecular analysis. I am happy to say that we were able to get ALK, EGFR & PDL1 testing on the EBUS-TBNA samples (where indicated), at our centre. The presence of a cytopathologist or cytotechnologist during the procedure for ROSE purposes can increase the cost significantly. This increased cost can have a significant impact on starting the service at the level of a district general hospital. Another issue which needs clarification, is the number of passes required before declaring the material is inadequate while using ROSE technique. Studies have shown that significant number of samples inadequate on ROSE were still able to give a diagnosis with the help of immunohistochemical analysis.
Here we have seen that 40 EBUS-TBNA procedures were carried out for IMHL. Unfortunately, in this group, one patient was diagnosed with unexpected malignancy, i.e., metastatic adenocarcinoma of pancreaticobiliary origin. In the remaining cases we had benign diagnoses. In the IMHL group about 45% cases had the diagnosis of reactive lymphadenopathy. Out of the total number of 82, about 33% cases were diagnosed with reactive lymphadenopathy. We made the diagnosis of reactive lymphadenopathy in patients where EBUS samples showed normal lymphocytes; these patients had surveillance CTs & clinical follow up as well. Clinicians’ impression & surveillance scans were also reviewed for the purpose of this diagnosis. In this IMHL group, 40% cases were diagnosed with sarcoidosis. In these cases, in addition to clinicians’ impressions, we reviewed cytology, microbiology & surveillance CT reports. Processing method for specimens impacts on the yield for granulomas. Cell block preparation, as carried out in this hospital, showed higher yield for granulomas 10.
During the first year of the EBUS service at this centre, there was no suspected or diagnosed lymphoma patient who underwent this procedure. International data suggests, for the diagnosis of lymphoma, EBUS-TBNA aspirates should be sent for cytopathology, immunohistochemistry, flow cytometry, cytogenetics and molecular studies 11,12,13.
Conclusion
EBUS-TBNA is a safe & minimally invasive procedure. It is a first line investigation for lung cancer staging. EBUS-TBNA has been effective in diagnosing extra-pulmonary malignancies 14. In the last decade we have also seen that its utility has increased significantly in diagnosing benign conditions like sarcoidosis and TB.
We feel operators’ training is also very important in achieving excellent results. Mastering the complexity of this procedure is time consuming. Standardised training is mandatory to achieve high skill levels15 and we hope there will be a standardised approach to this in future.
|
Competing Interests None declared Author Details AMER SALEEM, BSc MBBS MCPS MRCP FCPS FRCP FCCP, Speciality Certificate in Respiratory Medicine (RCP UK) & European Diploma in Adult Respiratory Medicine, Department of Respiratory Medicine, Milton Keynes University Hospital, Standing Way, Eaglestone, Milton Keynes MK6 5LD, UK. NEAL NAVANI, MA MSc PhD FRCP, Department of Thoracic Medicine, UCLH, 250-Euston Road London NW1 2PG, UK. VIVEK VOHRA, MD DMRD FRCR FFRRCSI, Department of Radiology, Bedford Hospital NHS Trust (South Wing), Kempston Road, Bedford MK42 9DJ, UK. CORRESPONDENCE: AMER SALEEM, Department of Respiratory Medicine Milton Keynes University Hospital, Standing Way, Eaglestone, Milton Keynes MK6 5LD, UK. Email: amersaleemmalik@gmail.com |
References
- Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009; 361:32-39.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011; 61:69-90.
- National Lung Cancer Audit Report, 2013.
- Navani N, Nankivell M, Lawrence D, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. The Lancet. 2015;3(4):282-289.
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systemic review and meta-analysis. Eur J Cancer. 2009; 45:1389-1396.
- British Thoracic Society. Quality Standards for Flexible Bronchoscopy in Adults. British Thoracic Society Reports 2014;6(5).
- Trisolini R, Cancellieri A, Tinelli C, Paioli D, Scudeller L, Casadei GP, Parri SF, Livi V, Bondi A, Boaron M, Patelli M. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest. 2011; 139:395-401.
- Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the Diagnostic Yield of Transbronchial Needle Aspiration During Mediastinal Lymph Node Sampling: systemic review and meta-analysis. Chest. 2018; 153(4):929-938.
- Navani N, Brown JM, Nankivell M, Woolhouse I, Harrison RN, Jeebun V, Munavvar M, Ng BJ, Rassl DM, Falzon M, Kocjan G, Rintoul RC, Nicholson AG, Janes SM: Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicentre study of 774 patients. Am J Respir Crit Care Med. 2012; 185:1316-1322.
- Schwartz LE, Griffin AC, Baloch Z. Cell block interpretation is helpful in the diagnosis of granulomas on cytology. Diagn Cytopathol. 2012; 40(10):939-40.
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis and subtyping of lymphoma. Ann Am Thorac Soc. 2015; 12:1336-1344.
- Soldini D, Campo E. New insights into the diagnosis of lymphomas. Ann Oncol. 2012; 23:83-88.
- Ko HM, daCunha Santos G, Darling G, et al. Diagnosis and subclassification of lymphomas and non-neoplastic lesions involving mediastinal lymph nodes using endobronchial ultrasound-guided transbronchial needle aspiration. Diagn Cytopathol. 2013; 41:1023-1030.
- Jain D. EBUS-TBNA for diagnosis of extrapulmonary lesions. J Cytol. 2019; 36(1):59-60.
- Zhang WC, Chen W, Zhou JP, et al. A comparison of different training methods in the successful learning of endobronchial ultrasound-guided transbronchial needle aspiration. Respiration. 2017; 93:319-326.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




