Hyperglycaemia and Myocardial Infarction
Sharan Badiger
Cite this article as: BJMP 2019;12(2):a010
|
Introduction
Hyperglycaemia is a condition in which an excessive amount of glucose circulates in the blood plasma. The origin of the term is Greek: hyper-, meaning excessive; -glyc-, meaning sweet; and -aemia, meaning "of the blood". Hyperglycaemia, or high blood glucose, is a serious health problem for those with diabetes. Normal fasting glucose is <100 mg/dl, impaired fasting glucose is 100–125 mg/dl, and diabetes mellitus is defined as a fasting glucose >126 mg/dl 1. Several values above normal are indicated before making a diagnosis of impaired fasting glucose or diabetes. Two types of hyperglycaemia can be seen in diabetic patients, they are, fasting hyperglycaemia defined as a blood sugar greater than 126 mg/dl after fasting for at least 8 hours and postprandial or after-meal hyperglycaemia defined as a blood sugar usually greater than 180 mg/dl. In people without diabetes postprandial or post-meal sugars rarely go over 140 mg/dl but occasionally, after a large meal, a 1-2 hour post-meal glucose level can reach 180 mg/dl.
Consistently elevated high post-meal glucose levels can be an indicator that a person is at high risk for developing type 2 diabetes. Stress hyperglycaemia also called as stress diabetes or diabetes of injury is a medical term referring to transient elevation of the blood glucose due to the stress of illness. It usually resolves spontaneously, but must be distinguished from various forms of diabetes mellitus. It is often discovered when routine blood chemistry measurements in an ill patient reveal an elevated blood glucose. Blood glucose can be assessed either by a bedside ‘fingerstick’ glucose meter or plasma glucose as performed in a laboratory. The glucose is typically in the range of 140-300 mg/dl but occasionally can exceed 500 mg/dl especially if amplified by drugs or intravenous glucose. The blood glucose usually returns to normal within hours unless predisposing drugs and intravenous glucose are continued.
Stress hyperglycaemia is especially common in patients with hypertonic dehydration and those with elevated catecholamine levels. Steroid diabetes is a specific and prolonged form of stress hyperglycaemia. In some people, stress hyperglycaemia may indicate a reduced insulin secretory capacity or a reduced sensitivity, and is sometimes the first clue to incipient diabetes (do you mean insipidus diabetes). Because of this, it is occasionally appropriate to perform diabetes screening tests after recovery from an illness in which significant stress hyperglycaemia occurred Table 1.
Table 1: Aetiology of Hyperglycaemia
| Glucose Tolerance Test | Impaired fasting glucose |
| Medications | Corticosteroids, growth hormone, estrogen, oral contraceptives, nicotinic acid, salicylates, NSAIDs, thiazide, loop diuretics, phenytoin, epinephrine |
| Diabetes mellitus | Diabetes mellitus type I, Diabetic ketoacidosis, Diabetes mellitus type II, Gestational diabetes |
| Pancreatic disease | Acute or chronic pancreatitis, Pancreatectomy, Pancreatic carcinoma, Haemochromatosis, Cystic fibrosis |
| Increased counter-regulatory hormones | Myocardial infarction, Stroke or other neurological disease, Renal insufficiency, Hepatic insufficiency |
| Endocrine disorders | Acromegaly, Cushing's syndrome, Pheochromocytoma, Hyperthyroidism (thyroid storm), Glucagonoma |
| Others | Amyloidosis |
Prevalence and risk of hyperglycemia
Acute hyperglycaemia is common in patients with ST- elevation myocardial infarction (STEMI) even in the absence of a history of type 2 diabetes mellitus (DM). Hyperglycaemia is encountered in up to 50% of all STEMI patients, whereas previously diagnosed DM is present in only 20% to 25% of STEMI patients 2 .The prevalence of type 2 DM or impaired glucose tolerance may be as high as 65% in myocardial infarction patients without prior DM when oral glucose tolerance testing is performed 3. Elevated plasma glucose and glycated haemoglobin levels on admission are independent prognosticators of both in-hospital and long-term outcome regardless of diabetic status 4, 5. For every 18-mg/dl increase in glucose level, there is a 4% increase in mortality in nondiabetic subjects 6. When admission glucose level exceeds 200 mg/dl, mortality is similar in non-DM and DM subjects with myocardial infarction (MI). Admission glucose has been identified as a major independent predictor of both in-hospital congestive heart failure and mortality in STEMI 7.
Fasting glucose the day after admission appears to be a better predictor of early mortality than glucose level on admission 8. Patients with both an elevated admission glucose and an elevated fasting glucose the next day have a 3-fold increase in mortality. Similarly, failure of an elevated glucose level to fall within 24 hours of admission is associated with excess mortality in STEMI patients without DM 9. The presence and degree of hyperglycaemia may not correlate with infarct size, as is commonly thought 6. Counter regulatory hormones like catecholamine, growth hormone, glucagons and cortisol are released in proportion to the degree of cardiovascular stress and may cause hyperglycemia and an elevation of free fatty acids, both of which lead to an increase in hepatic gluconeogenesis and a decrease in insulin-mediated peripheral glucose disposal.
Pathogenesis of hyperglycaemia
When acute coronary artery occlusion leads to symptoms, aid this is not always the case, there is stimulation of postganglionic sympathetic nerve endings with release of norepinephrine, and of the adrenal medulla with release of epinephrine. Both catecholamines are present in high concentrations in plasma and urine during the first 24-48 hours after the onset of symptoms. The concentrations of these catecholamines in plasma reach high levels within the first few hours after the onset of symptoms and later appear to be related to the severity of the infarct. Norepinephrine acts through beta-adrenergic receptors, to activate the adenylcyclase system in adipose tissue causing conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (AMP) and cyclic AMP activates a lipolytic system leading to hydrolysis of stored triglycerides to diglycerides, free fatty acids (FFA) and also glycerol. While some reesterification of FFA occurs, the net effect is release of FFA and glycerol into the circulation. In acute myocardial infarction, plasma FFA concentrations are elevated within 4 hours of the onset of symptoms. The highest values are found on the first day, and by the sixth day normal values are usually reached.
Glycerol levels are also elevated. There is a close relationship between blood catecholamine and FFA values in myocardial infarction. Epinephrine has a weak effect on adipose tissue lipolysis but its main action at this time is to stimulate glycogenolysis in liver and muscle with elevation of blood glucose levels. Epinephrine also suppresses beta cell activity in the pancreas with a decrease in insulin secretion leading to further elevation of blood glucose. Thus, hyperglycaemia occurs after acute myocardial infarction, and more than half of these patients have an abnormal glucose tolerance test during the first 72 hours of the attack. Reduction of insulin secretion has been demonstrated in patients after acute myocardial infarction following an intravenous glucose load and an intravenous Tolbutamide test. The degree of failure in these responses has been positively correlated with the severity of the illness and with the presence of cardiogenic shock.
Cortisol secretion and plasma growth hormone levels are increased during the first 24 hours after the onset of acute myocardial infarction. As the clinical condition improves, the degree of glucose intolerance diminishes and insulin secretion increases. In the second week plasma insulin levels are above normal, and at this stage the anabolic effect of insulin in enhancing the transport of amino acids into cells and their incorporation into protein is important for repair of the injured myocardium. Cortisol stimulates the breakdown of protein for gluconeogenic purposes and also the key gluconeogenic enzymes, but it is doubtful whether these actions operate until the acute period has passed 10, Figure 1.
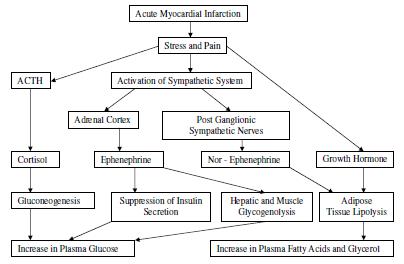
Figure 1
Cardiovascular effects of hyperglycaemia
Acute hyperglycaemia is associated with numerous adverse effects that contribute to a poor outcome in STEMI. Acute hyperglycaemia rapidly suppresses flow-mediated vasodilatation, likely through increased production of oxygen derived free radicals 11. Hyperglycaemia increases intranuclear nuclear factor-B binding and activates proinflammatory transcription factors, which increase the expression of matrix metalloproteinase, tissue factor, and plasminogen activator inhibitor-1. The degree of oxidative stress correlates most closely with acute, not chronic, glucose fluctuations 12.
Increased oxidative stress interferes with nitric oxide mediated vasodilatation and reduces coronary blood flow at the micro vascular level. In STEMI subjects, acute hyperglycaemia is associated with reduced TIMI grade 3 flow before intervention compared with euglycemia and is the most important predictor of the absence of coronary perfusion 13. Similarly, diabetic subjects have reduced myocardial blush grades and diminished ST-segment resolution after successful coronary intervention in STEMI, consistent with diminished micro vascular perfusion 14.
Acute hyperglycaemia is associated with impaired microcirculatory function as manifest by “no reflow” on myocardial contrast echocardiography after percutaneous coronary intervention 15. Pre-existing HbA1c levels and diabetes status do not differ between subsets with and without no reflow, suggesting that acute, not chronic, hyperglycaemia is the dominant factor. Finally, the well-known adverse effects of hyperglycaemia on platelet function, fibrinolysis, coagulation, and ischaemic preconditioning likely contribute to the adverse effects of acute hyperglycaemia in STEMI. Hyperglycaemia is a reflection of relative insulinopenia, which is associated with increased lipolysis and free fatty acid generation, as well as diminished myocardial glucose uptake and a decrease in glycolytic substrate for myocardial energy needs in STEMI. Myocardial ischemia results in an increased rate of glycogenolysis and glucose uptake via translocation of GLUT-4 receptors to the sarcolemmal 16. Because glucose oxidation requires less oxygen than free fatty acid oxidation per molecule of ATP produced, myocardial energetics are more efficient during the increased dependence on glucose oxidation with ischaemia.
With relative insulinopenia, however, the ischaemic myocardium is forced to use free fatty acids instead of glucose as an energy source because myocardial glucose uptake is acutely impaired. Thus, a metabolic crisis may ensue as the hypoxic myocardium becomes less energy efficient in the setting of hyperglycaemia and insulin resistance. Acute hyperglycaemia may precipitate an osmotic diuresis. The resulting volume depletion may interfere with the frank starling mechanism for the failing left ventricle in which increased end diastolic volume leads to increased stroke volume thus decreasing the cardiac output 17, Table 2.
Table 2: Acute Cardiovascular Effects of Hyperglycaemia
| Endothelial dysfunction |
| Platelet hyperreactivity |
| Increased cytokine activation |
| Reduced glycolysis and glucose oxidation |
| Increased lipolysis and free fatty acid levels |
| Increased oxidative stress (? Increased myocardial apoptosis) |
| Impaired microcirculatory function (“no-reflow” phenomenon) |
| Impaired ischaemic preconditioning |
| Impaired insulin secretion and insulin based glucose uptake |
Conclusion
An essential diagnostic feature of diabetes is increased blood glucose concentration and the principal aim of diabetes treatment is normalisation of blood glucose. Hyperglycaemia can also occur when normal hormonal control of blood glucose concentration is disturbed by the stress associated with acute myocardial infarction.
The blood glucose is raised in the immediate period following acute myocardial infarction irrespective of diabetes status. In this review article the current understanding of the significance of hyperglycaemia occurring as a result of acute myocardial infarction is discussed.
A significant part of the review is directed to the discussion of epidemiological prevalence that confirms an association between hyperglycaemia and mortality following myocardial infarction.
It remains clear and undisputed that there is association between hyperglycaemia and increased mortality following acute myocardial infarction. Review of various articles ranging from experimental and clinical studies have demonstrated several mechanisms by which hyperglycaemia could adversely affect outcome of myocardial infarction. The final part of the review concluded that if treatment is aimed at normalising blood glucose improves outcome of acute myocardial infarction patients who present with hyperglycaemia.
|
Competing Interests None declared Author Details SHARAN BADIGER, MD, Professor, Department of Medicine, Sri B M Patil Medical College, Vijayapur, Karnataka, India. CORRESPONDENCE: SHARAN BADIGER, MD, Professor, Department of Medicine, Sri B M Patil Medical College, Vijayapur, Karnataka, India. Email: sharanrb@rediffmail.com |
References
- Avel C. Powers Diabetes Mellitus In: Kasper, Braunwald, Fauci Hauser, Longo, and Jameson editors. Harrison’s Principles of Internal Medicine, Vol-2.17th Ed; Newyork: McGraw Hill; 2005; 2126-2127.
- Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002; 40:1748 –1754.
- Norhammar A, Tenerz A, Nilsson G, Hansten A, efendic S, Ryden L, Malmberg K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002; 359:2140 –2144.
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000; 355:773–778.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation.1999; 99: 2626 –2632.
- Stranders I, Diamant M, van Gelder R, Spruijt H, Twisk JWR, Heine RJ, Visser FC. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004; 164:982–988.
- Zeller M, Steg P, Ravisy J, Laurent Y, Janin- Manificat L, L’Huillier I, Beer J, Oudot A, Rioufol G, Makki H, Farnier M, Rochette L, Verges, Cottin Y. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch Intern Med. 2005; 165:1192–1198
- Suleiman M, Hammerman H, Boulos M, Kapeliovich M, Suleiman A, Agmon Y, Markiewicz W, Aronson D. Fasting glucose is an important independent risk factor for 30-day mortality in patients with acute myocardial infarction. Circulation. 2005; 111:754-760
- Goyal A, Mahaffey K, Garg J, Nicolau JC, Hochman JS, Weaver WD, Theroux P, Oliveira GBF, Todaro TG, Mojcik CF, Armstrong PW, Granger CB. Prognostic significance of the change in glucose level in the first 24h after acute myocardial infarction: results from the CARDINAL study.EurHeartJ.2006; 27:1289 –1297.
- Oliver MF. Metabolic Response during Impending Myocardial Infarction. Clinical implications II Circulation 1972; 45; 491-500
- Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T,Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium- dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999; 34:146 –154.
- Monnier L, Mas E, Ginet C, Michel F, Willon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–1687.
- Timmer J, Ottervanger J, de Boer M, Dambrink JE, Hoorntje JCA, Gosselink ATM, Suryapranata H, Zijlstra F, Van’t Hof AWJ, for the Zwolle Myocardial Infarction Study Group. Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2005; 45:999 –1002.
- Prasad A, Stone G, Stuckey T, Costantini CO, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox DA, Grines CL, Lansky AJ, Gersh BJ. Impact of diabetes mellitus on myocardial perfusion after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol. 45:508 –514.
- Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, Kuroda T, Tanaka K, Masuyama T, Hori M, Fujii K. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003; 41:1–7.
- Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, Shulman GI, Sinusas AJ. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997; 95:415– 422.
- Sarah E Capes, Dereck Hunt, KlasMalmberg, Hertzel C Gerstein. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: Lancet 2000; 355:773-777.

The above article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.




